321827
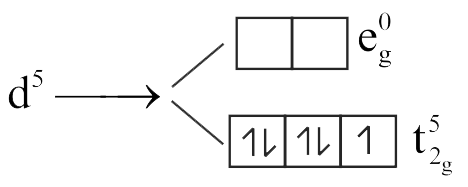
Give the electronic configuration of the following complexes on the basis of Crystal Field Splitting theory.
\(\left[\mathrm{CoF}_{6}\right]^{3-},\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}\) and \(\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\)
321827
Give the electronic configuration of the following complexes on the basis of Crystal Field Splitting theory.
\(\left[\mathrm{CoF}_{6}\right]^{3-},\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}\) and \(\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\)
321827
Give the electronic configuration of the following complexes on the basis of Crystal Field Splitting theory.
\(\left[\mathrm{CoF}_{6}\right]^{3-},\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}\) and \(\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\)
321827
Give the electronic configuration of the following complexes on the basis of Crystal Field Splitting theory.
\(\left[\mathrm{CoF}_{6}\right]^{3-},\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}\) and \(\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\)