321630
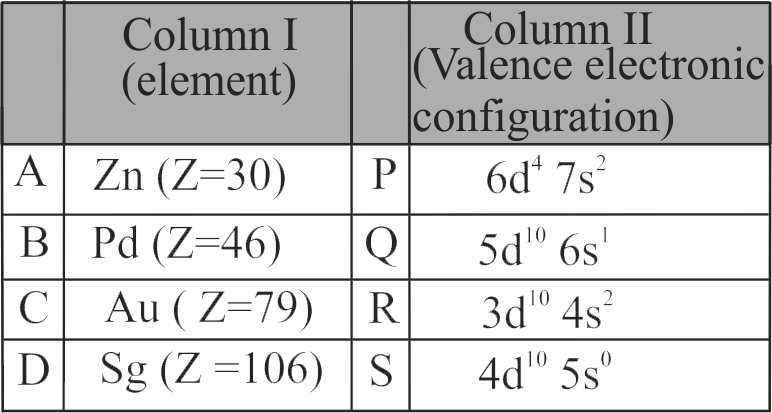
Match Column I (Species) with Column II (Electronic distribution).
Column I
Column II
A
\({\rm{C}}{{\rm{r}}^{{\rm{2 + }}}}\)
P
\({\rm{3}}{{\rm{d}}^{\rm{8}}}\)
B
\({\rm{M}}{{\rm{n}}^{\rm{ + }}}\)
Q
\({\rm{3}}{{\rm{d}}^{\rm{3}}}{\rm{4}}{{\rm{s}}^{\rm{1}}}\)
C
\({\rm{N}}{{\rm{i}}^{{\rm{2 + }}}}\)
R
\({\rm{3}}{{\rm{d}}^4}\)
D
\({{\rm{V}}^{\rm{ + }}}\)
S
\({\rm{3}}{{\rm{d}}^{\rm{5}}}{\rm{4}}{{\rm{s}}^{\rm{1}}}\)
321630
Match Column I (Species) with Column II (Electronic distribution).
Column I
Column II
A
\({\rm{C}}{{\rm{r}}^{{\rm{2 + }}}}\)
P
\({\rm{3}}{{\rm{d}}^{\rm{8}}}\)
B
\({\rm{M}}{{\rm{n}}^{\rm{ + }}}\)
Q
\({\rm{3}}{{\rm{d}}^{\rm{3}}}{\rm{4}}{{\rm{s}}^{\rm{1}}}\)
C
\({\rm{N}}{{\rm{i}}^{{\rm{2 + }}}}\)
R
\({\rm{3}}{{\rm{d}}^4}\)
D
\({{\rm{V}}^{\rm{ + }}}\)
S
\({\rm{3}}{{\rm{d}}^{\rm{5}}}{\rm{4}}{{\rm{s}}^{\rm{1}}}\)
321630
Match Column I (Species) with Column II (Electronic distribution).
Column I
Column II
A
\({\rm{C}}{{\rm{r}}^{{\rm{2 + }}}}\)
P
\({\rm{3}}{{\rm{d}}^{\rm{8}}}\)
B
\({\rm{M}}{{\rm{n}}^{\rm{ + }}}\)
Q
\({\rm{3}}{{\rm{d}}^{\rm{3}}}{\rm{4}}{{\rm{s}}^{\rm{1}}}\)
C
\({\rm{N}}{{\rm{i}}^{{\rm{2 + }}}}\)
R
\({\rm{3}}{{\rm{d}}^4}\)
D
\({{\rm{V}}^{\rm{ + }}}\)
S
\({\rm{3}}{{\rm{d}}^{\rm{5}}}{\rm{4}}{{\rm{s}}^{\rm{1}}}\)
321630
Match Column I (Species) with Column II (Electronic distribution).
Column I
Column II
A
\({\rm{C}}{{\rm{r}}^{{\rm{2 + }}}}\)
P
\({\rm{3}}{{\rm{d}}^{\rm{8}}}\)
B
\({\rm{M}}{{\rm{n}}^{\rm{ + }}}\)
Q
\({\rm{3}}{{\rm{d}}^{\rm{3}}}{\rm{4}}{{\rm{s}}^{\rm{1}}}\)
C
\({\rm{N}}{{\rm{i}}^{{\rm{2 + }}}}\)
R
\({\rm{3}}{{\rm{d}}^4}\)
D
\({{\rm{V}}^{\rm{ + }}}\)
S
\({\rm{3}}{{\rm{d}}^{\rm{5}}}{\rm{4}}{{\rm{s}}^{\rm{1}}}\)