321549
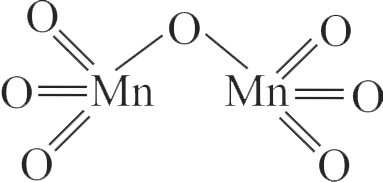
Highest oxidation state of Mn is exhibited in \({\rm{M}}{{\rm{n}}_2}{{\rm{O}}_7}.\) The correct statements about \(\mathrm{Mn}_{2} \mathrm{O}_{7}\) are
A. Mn is tetrahedrally surrounded by oxygen atoms.
B. Mn is octahedrally surrounded by oxygen atoms.
C. Contains Mn-O-Mn bridge.
D. Contains Mn-Mn bond.
Choose the correct answer from the options given below:
321549
Highest oxidation state of Mn is exhibited in \({\rm{M}}{{\rm{n}}_2}{{\rm{O}}_7}.\) The correct statements about \(\mathrm{Mn}_{2} \mathrm{O}_{7}\) are
A. Mn is tetrahedrally surrounded by oxygen atoms.
B. Mn is octahedrally surrounded by oxygen atoms.
C. Contains Mn-O-Mn bridge.
D. Contains Mn-Mn bond.
Choose the correct answer from the options given below:
321549
Highest oxidation state of Mn is exhibited in \({\rm{M}}{{\rm{n}}_2}{{\rm{O}}_7}.\) The correct statements about \(\mathrm{Mn}_{2} \mathrm{O}_{7}\) are
A. Mn is tetrahedrally surrounded by oxygen atoms.
B. Mn is octahedrally surrounded by oxygen atoms.
C. Contains Mn-O-Mn bridge.
D. Contains Mn-Mn bond.
Choose the correct answer from the options given below:
321549
Highest oxidation state of Mn is exhibited in \({\rm{M}}{{\rm{n}}_2}{{\rm{O}}_7}.\) The correct statements about \(\mathrm{Mn}_{2} \mathrm{O}_{7}\) are
A. Mn is tetrahedrally surrounded by oxygen atoms.
B. Mn is octahedrally surrounded by oxygen atoms.
C. Contains Mn-O-Mn bridge.
D. Contains Mn-Mn bond.
Choose the correct answer from the options given below: