321486
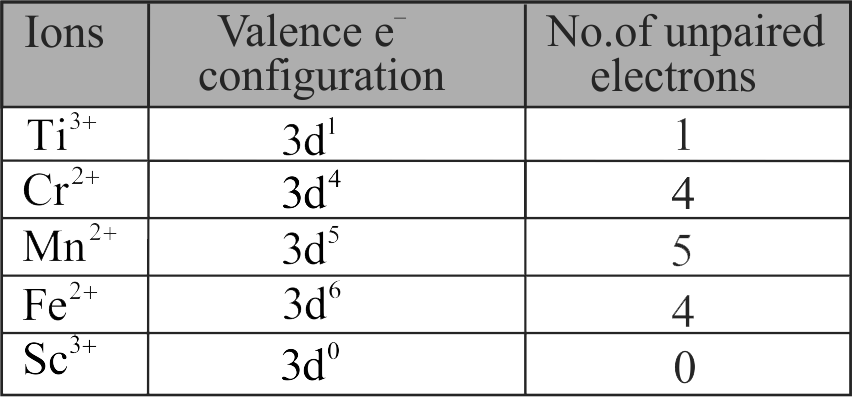
Spin only ' magnetic moment is same for which of the following ions?
(A) \({\mathrm{\mathrm{Ti}^{3+}}}\)(B) \({\mathrm{\mathrm{Cr}^{2+}}}\)(C) \({\mathrm{\mathrm{Mn}^{2+}}}\)(D) \({\mathrm{\mathrm{Fe}^{2+}}}\)(E) \({\mathrm{\mathrm{Sc}^{3+}}}\)
Choose the most appropriate answer from the options given below.
321486
Spin only ' magnetic moment is same for which of the following ions?
(A) \({\mathrm{\mathrm{Ti}^{3+}}}\)(B) \({\mathrm{\mathrm{Cr}^{2+}}}\)(C) \({\mathrm{\mathrm{Mn}^{2+}}}\)(D) \({\mathrm{\mathrm{Fe}^{2+}}}\)(E) \({\mathrm{\mathrm{Sc}^{3+}}}\)
Choose the most appropriate answer from the options given below.
321486
Spin only ' magnetic moment is same for which of the following ions?
(A) \({\mathrm{\mathrm{Ti}^{3+}}}\)(B) \({\mathrm{\mathrm{Cr}^{2+}}}\)(C) \({\mathrm{\mathrm{Mn}^{2+}}}\)(D) \({\mathrm{\mathrm{Fe}^{2+}}}\)(E) \({\mathrm{\mathrm{Sc}^{3+}}}\)
Choose the most appropriate answer from the options given below.
321486
Spin only ' magnetic moment is same for which of the following ions?
(A) \({\mathrm{\mathrm{Ti}^{3+}}}\)(B) \({\mathrm{\mathrm{Cr}^{2+}}}\)(C) \({\mathrm{\mathrm{Mn}^{2+}}}\)(D) \({\mathrm{\mathrm{Fe}^{2+}}}\)(E) \({\mathrm{\mathrm{Sc}^{3+}}}\)
Choose the most appropriate answer from the options given below.