321476
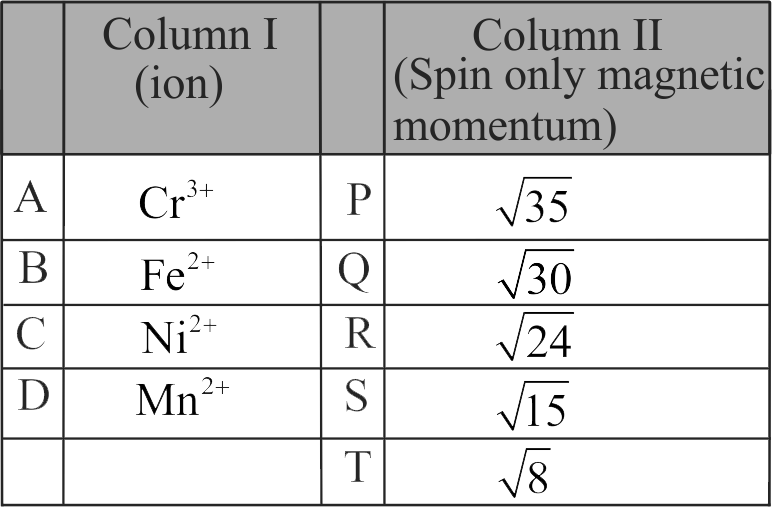
Match the metal ions given in Column I with the spin magnetic moments of the ions given in Column II and assign the correct code :
Column I
Column II
A
\(\rm{C{o^{3 + }}}\)
P
\(\rm{\sqrt 8 \;B.M}\)
B
\(\rm{C{r^{3 + }}}\)
Q
\(\rm{\sqrt {35} \;B.M}\)
C
\(\rm{F{e^{3 + }}}\)
R
\(\rm{\sqrt 3 \;B.M}\)
D
\(\rm{N{i^{2 + }}}\)
S
\(\rm{\sqrt {24} \;B.M}\)
T
\(\rm{\sqrt {15} \;B.M}\)
321476
Match the metal ions given in Column I with the spin magnetic moments of the ions given in Column II and assign the correct code :
Column I
Column II
A
\(\rm{C{o^{3 + }}}\)
P
\(\rm{\sqrt 8 \;B.M}\)
B
\(\rm{C{r^{3 + }}}\)
Q
\(\rm{\sqrt {35} \;B.M}\)
C
\(\rm{F{e^{3 + }}}\)
R
\(\rm{\sqrt 3 \;B.M}\)
D
\(\rm{N{i^{2 + }}}\)
S
\(\rm{\sqrt {24} \;B.M}\)
T
\(\rm{\sqrt {15} \;B.M}\)
321476
Match the metal ions given in Column I with the spin magnetic moments of the ions given in Column II and assign the correct code :
Column I
Column II
A
\(\rm{C{o^{3 + }}}\)
P
\(\rm{\sqrt 8 \;B.M}\)
B
\(\rm{C{r^{3 + }}}\)
Q
\(\rm{\sqrt {35} \;B.M}\)
C
\(\rm{F{e^{3 + }}}\)
R
\(\rm{\sqrt 3 \;B.M}\)
D
\(\rm{N{i^{2 + }}}\)
S
\(\rm{\sqrt {24} \;B.M}\)
T
\(\rm{\sqrt {15} \;B.M}\)
321476
Match the metal ions given in Column I with the spin magnetic moments of the ions given in Column II and assign the correct code :
Column I
Column II
A
\(\rm{C{o^{3 + }}}\)
P
\(\rm{\sqrt 8 \;B.M}\)
B
\(\rm{C{r^{3 + }}}\)
Q
\(\rm{\sqrt {35} \;B.M}\)
C
\(\rm{F{e^{3 + }}}\)
R
\(\rm{\sqrt 3 \;B.M}\)
D
\(\rm{N{i^{2 + }}}\)
S
\(\rm{\sqrt {24} \;B.M}\)
T
\(\rm{\sqrt {15} \;B.M}\)