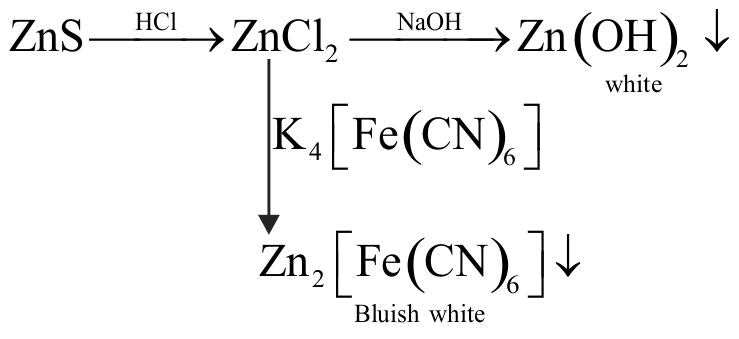321398 When \(\mathrm{\mathrm{XO}_{2}}\) is used with an alkali metal hydroxide in presence of an oxidizing agent such as \(\mathrm{\mathrm{KNO}_{3}}\); a dark green product is formed which disproportionates in acidic solution to afford a dark purple solution. \(\mathrm{\mathrm{X}}\) is:
321400 A solution containing a group - IV cation gives a precipitate on passing, \({{\rm{H}}_{\rm{2}}}{\rm{S}}\). A solution of this precipitate in dil. HCI produces a white precipitate with \(\mathrm{\mathrm{NaOH}}\) solution and bluish- white prcipitate with basic potassium ferrocyanide. The cation is :
321398 When \(\mathrm{\mathrm{XO}_{2}}\) is used with an alkali metal hydroxide in presence of an oxidizing agent such as \(\mathrm{\mathrm{KNO}_{3}}\); a dark green product is formed which disproportionates in acidic solution to afford a dark purple solution. \(\mathrm{\mathrm{X}}\) is:
321400 A solution containing a group - IV cation gives a precipitate on passing, \({{\rm{H}}_{\rm{2}}}{\rm{S}}\). A solution of this precipitate in dil. HCI produces a white precipitate with \(\mathrm{\mathrm{NaOH}}\) solution and bluish- white prcipitate with basic potassium ferrocyanide. The cation is :
321398 When \(\mathrm{\mathrm{XO}_{2}}\) is used with an alkali metal hydroxide in presence of an oxidizing agent such as \(\mathrm{\mathrm{KNO}_{3}}\); a dark green product is formed which disproportionates in acidic solution to afford a dark purple solution. \(\mathrm{\mathrm{X}}\) is:
321400 A solution containing a group - IV cation gives a precipitate on passing, \({{\rm{H}}_{\rm{2}}}{\rm{S}}\). A solution of this precipitate in dil. HCI produces a white precipitate with \(\mathrm{\mathrm{NaOH}}\) solution and bluish- white prcipitate with basic potassium ferrocyanide. The cation is :
321398 When \(\mathrm{\mathrm{XO}_{2}}\) is used with an alkali metal hydroxide in presence of an oxidizing agent such as \(\mathrm{\mathrm{KNO}_{3}}\); a dark green product is formed which disproportionates in acidic solution to afford a dark purple solution. \(\mathrm{\mathrm{X}}\) is:
321400 A solution containing a group - IV cation gives a precipitate on passing, \({{\rm{H}}_{\rm{2}}}{\rm{S}}\). A solution of this precipitate in dil. HCI produces a white precipitate with \(\mathrm{\mathrm{NaOH}}\) solution and bluish- white prcipitate with basic potassium ferrocyanide. The cation is :