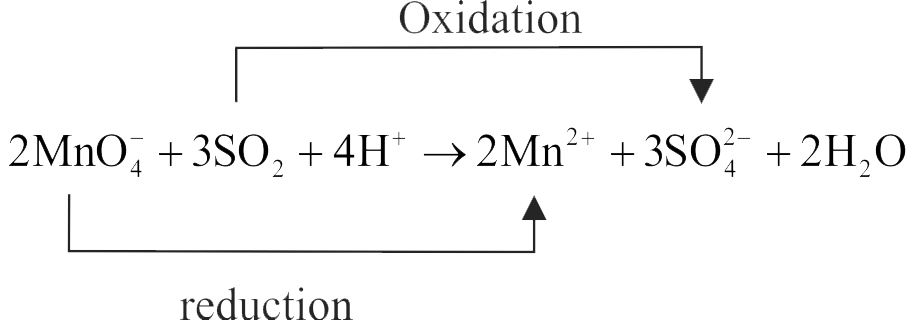
321347
\({\text{KMn}}{{\text{O}}_{\text{4}}}\xrightarrow[{{\text{R}}{\text{.A}}{\text{.}}}]{{{{\text{H}}^{\text{ + }}}}}{{\text{M}}^{{\text{x + }}}}\)
\({\text{KMn}}{{\text{O}}_{\text{4}}}\xrightarrow[{{\text{R}}{\text{.A}}}]{{{\text{0}}{{\text{H}}^{{\text{ - 1}}}}}}{\text{M}}{{\text{n}}^{{\text{y + }}}}\)
\({{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\xrightarrow{{{\text{O}}{{\text{H}}^{\text{ - }}}}}{\text{C}}{{\text{r}}^{{\text{z + }}}}\) \(\left( {{\text{x + y + z}}} \right)\) value is:
(here \(\mathrm{\mathrm{x}, \mathrm{y}}\) and \(\mathrm{\mathrm{z}}\) are oxidation states)
321347
\({\text{KMn}}{{\text{O}}_{\text{4}}}\xrightarrow[{{\text{R}}{\text{.A}}{\text{.}}}]{{{{\text{H}}^{\text{ + }}}}}{{\text{M}}^{{\text{x + }}}}\)
\({\text{KMn}}{{\text{O}}_{\text{4}}}\xrightarrow[{{\text{R}}{\text{.A}}}]{{{\text{0}}{{\text{H}}^{{\text{ - 1}}}}}}{\text{M}}{{\text{n}}^{{\text{y + }}}}\)
\({{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\xrightarrow{{{\text{O}}{{\text{H}}^{\text{ - }}}}}{\text{C}}{{\text{r}}^{{\text{z + }}}}\) \(\left( {{\text{x + y + z}}} \right)\) value is:
(here \(\mathrm{\mathrm{x}, \mathrm{y}}\) and \(\mathrm{\mathrm{z}}\) are oxidation states)
321347
\({\text{KMn}}{{\text{O}}_{\text{4}}}\xrightarrow[{{\text{R}}{\text{.A}}{\text{.}}}]{{{{\text{H}}^{\text{ + }}}}}{{\text{M}}^{{\text{x + }}}}\)
\({\text{KMn}}{{\text{O}}_{\text{4}}}\xrightarrow[{{\text{R}}{\text{.A}}}]{{{\text{0}}{{\text{H}}^{{\text{ - 1}}}}}}{\text{M}}{{\text{n}}^{{\text{y + }}}}\)
\({{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\xrightarrow{{{\text{O}}{{\text{H}}^{\text{ - }}}}}{\text{C}}{{\text{r}}^{{\text{z + }}}}\) \(\left( {{\text{x + y + z}}} \right)\) value is:
(here \(\mathrm{\mathrm{x}, \mathrm{y}}\) and \(\mathrm{\mathrm{z}}\) are oxidation states)
321347
\({\text{KMn}}{{\text{O}}_{\text{4}}}\xrightarrow[{{\text{R}}{\text{.A}}{\text{.}}}]{{{{\text{H}}^{\text{ + }}}}}{{\text{M}}^{{\text{x + }}}}\)
\({\text{KMn}}{{\text{O}}_{\text{4}}}\xrightarrow[{{\text{R}}{\text{.A}}}]{{{\text{0}}{{\text{H}}^{{\text{ - 1}}}}}}{\text{M}}{{\text{n}}^{{\text{y + }}}}\)
\({{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\xrightarrow{{{\text{O}}{{\text{H}}^{\text{ - }}}}}{\text{C}}{{\text{r}}^{{\text{z + }}}}\) \(\left( {{\text{x + y + z}}} \right)\) value is:
(here \(\mathrm{\mathrm{x}, \mathrm{y}}\) and \(\mathrm{\mathrm{z}}\) are oxidation states)