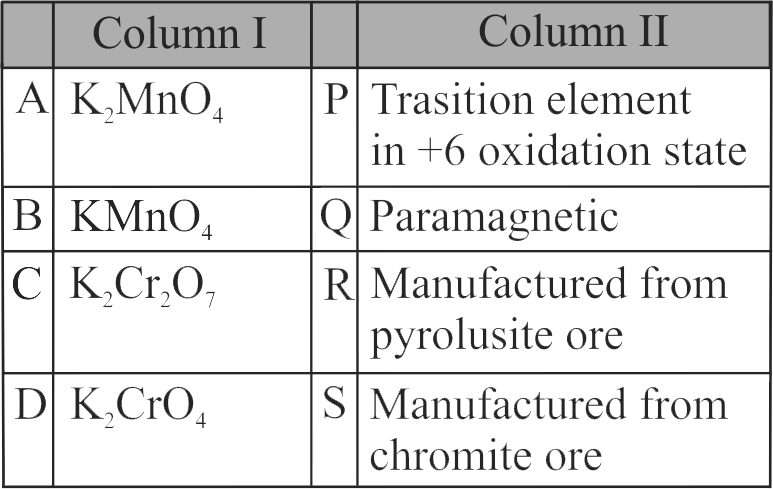
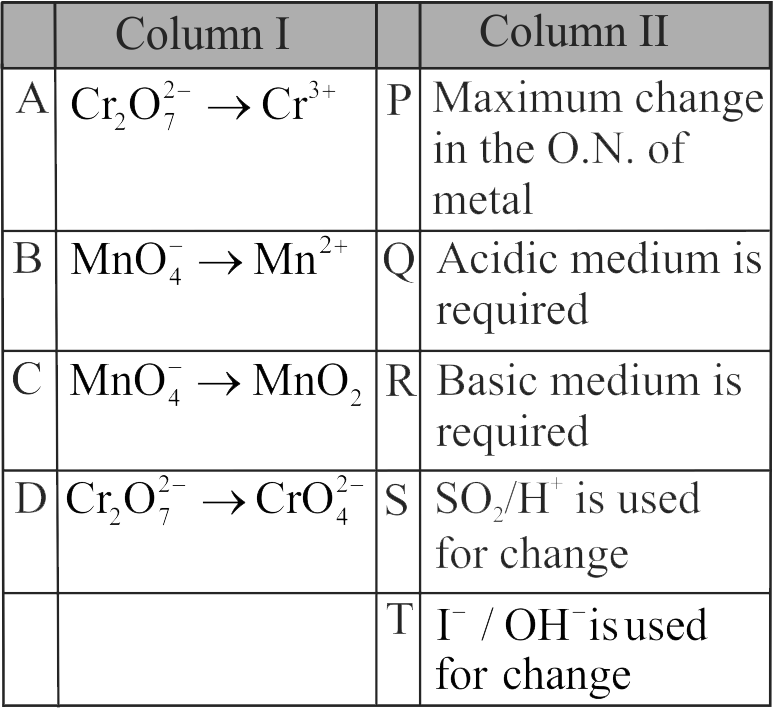
321340
Statement A :
Permanganate at \(\mathrm{\left[\mathrm{H}^{+}\right]=1}\) should oxidise water but in practice the reaction is extremely slow.
Statement B :
The above reaction is practically feasible only when either manganese (II) ions are present or the temperature is raised.
321340
Statement A :
Permanganate at \(\mathrm{\left[\mathrm{H}^{+}\right]=1}\) should oxidise water but in practice the reaction is extremely slow.
Statement B :
The above reaction is practically feasible only when either manganese (II) ions are present or the temperature is raised.
321340
Statement A :
Permanganate at \(\mathrm{\left[\mathrm{H}^{+}\right]=1}\) should oxidise water but in practice the reaction is extremely slow.
Statement B :
The above reaction is practically feasible only when either manganese (II) ions are present or the temperature is raised.
321340
Statement A :
Permanganate at \(\mathrm{\left[\mathrm{H}^{+}\right]=1}\) should oxidise water but in practice the reaction is extremely slow.
Statement B :
The above reaction is practically feasible only when either manganese (II) ions are present or the temperature is raised.
321340
Statement A :
Permanganate at \(\mathrm{\left[\mathrm{H}^{+}\right]=1}\) should oxidise water but in practice the reaction is extremely slow.
Statement B :
The above reaction is practically feasible only when either manganese (II) ions are present or the temperature is raised.