CHXII06:GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
321191
Although thermodynamically feasible, in practice, magnesium metal is not used for the reduction of alumina in the metallurgy of aluminium. Why?
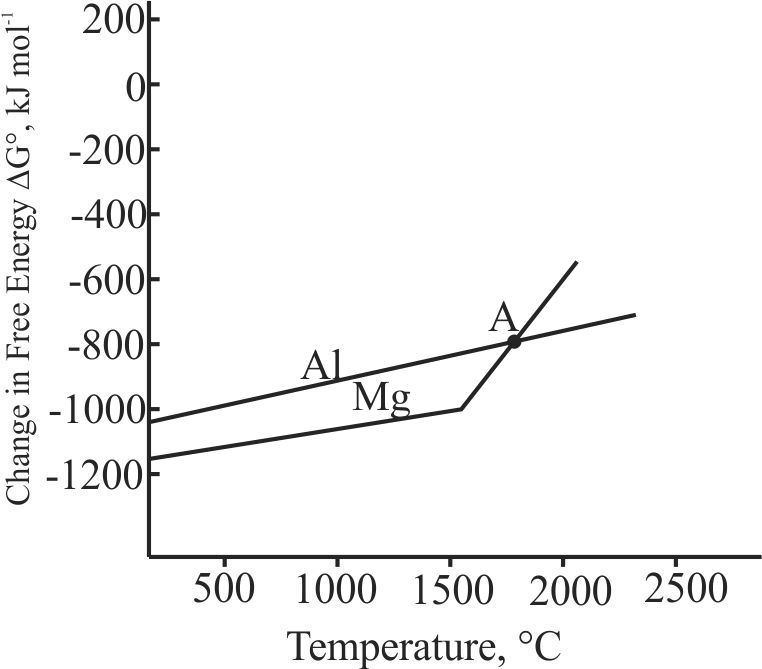
1 can reduce Alumina below point at temperature lower than at
2 The process is uneconomical
3 Though thermodynamically feasible practically not possible
4 Both (1) and (2)
Explanation:
Temperature below the point of intersection of and curves, magnesium can reduce alumina. But the process will be uneconomical.