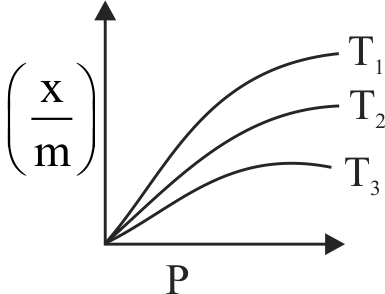
320832 Graph between \({\rm{log}}\frac{{\rm{x}}}{{\rm{m}}}\) and \(\log \mathrm{P}\) is a straight line inclined at an angle of \(45^{\circ}\). When pressure is \(0.5 \mathrm{~atm}\) and \(\ln \mathrm{k}=0.693\), the amount of solute adsorbed per gram of adsorbent will be :
320832 Graph between \({\rm{log}}\frac{{\rm{x}}}{{\rm{m}}}\) and \(\log \mathrm{P}\) is a straight line inclined at an angle of \(45^{\circ}\). When pressure is \(0.5 \mathrm{~atm}\) and \(\ln \mathrm{k}=0.693\), the amount of solute adsorbed per gram of adsorbent will be :
320832 Graph between \({\rm{log}}\frac{{\rm{x}}}{{\rm{m}}}\) and \(\log \mathrm{P}\) is a straight line inclined at an angle of \(45^{\circ}\). When pressure is \(0.5 \mathrm{~atm}\) and \(\ln \mathrm{k}=0.693\), the amount of solute adsorbed per gram of adsorbent will be :