320548
Assertion :
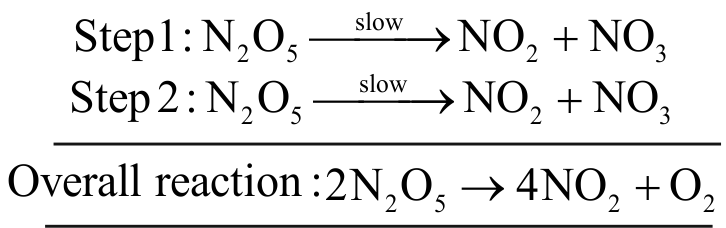
For the reaction,
\(2 \mathrm{~N}_{2} \mathrm{O}_{5} \rightarrow 4 \mathrm{NO}_{2}+\mathrm{O}_{2}\), Rate \(=\mathrm{k}\left[\mathrm{N}_{2} \mathrm{O}_{5}\right]\)
Reason :
Rate of decomposition of \(\mathrm{N}_{2} \mathrm{O}_{5}\) is determined by slow step.
320551
A certain reaction occurs in two steps as
(i) \({\rm{2S}}{{\rm{O}}_{\rm{2}}}{\rm{(g) + 2N}}{{\rm{O}}_{\rm{2}}}{\rm{(g)}} \to {\rm{2S}}{{\rm{O}}_{\rm{3}}}{\rm{(g) + }}\)
\(\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{2NO(g)}}\)
(ii) \(2 \mathrm{NO}(\mathrm{g})+\mathrm{O}_{2}(\mathrm{~g}) \longrightarrow 2 \mathrm{NO}_{2}(\mathrm{~g})\)
which of the following is correct?
320548
Assertion :
For the reaction,
\(2 \mathrm{~N}_{2} \mathrm{O}_{5} \rightarrow 4 \mathrm{NO}_{2}+\mathrm{O}_{2}\), Rate \(=\mathrm{k}\left[\mathrm{N}_{2} \mathrm{O}_{5}\right]\)
Reason :
Rate of decomposition of \(\mathrm{N}_{2} \mathrm{O}_{5}\) is determined by slow step.
320551
A certain reaction occurs in two steps as
(i) \({\rm{2S}}{{\rm{O}}_{\rm{2}}}{\rm{(g) + 2N}}{{\rm{O}}_{\rm{2}}}{\rm{(g)}} \to {\rm{2S}}{{\rm{O}}_{\rm{3}}}{\rm{(g) + }}\)
\(\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{2NO(g)}}\)
(ii) \(2 \mathrm{NO}(\mathrm{g})+\mathrm{O}_{2}(\mathrm{~g}) \longrightarrow 2 \mathrm{NO}_{2}(\mathrm{~g})\)
which of the following is correct?
320548
Assertion :
For the reaction,
\(2 \mathrm{~N}_{2} \mathrm{O}_{5} \rightarrow 4 \mathrm{NO}_{2}+\mathrm{O}_{2}\), Rate \(=\mathrm{k}\left[\mathrm{N}_{2} \mathrm{O}_{5}\right]\)
Reason :
Rate of decomposition of \(\mathrm{N}_{2} \mathrm{O}_{5}\) is determined by slow step.
320551
A certain reaction occurs in two steps as
(i) \({\rm{2S}}{{\rm{O}}_{\rm{2}}}{\rm{(g) + 2N}}{{\rm{O}}_{\rm{2}}}{\rm{(g)}} \to {\rm{2S}}{{\rm{O}}_{\rm{3}}}{\rm{(g) + }}\)
\(\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{2NO(g)}}\)
(ii) \(2 \mathrm{NO}(\mathrm{g})+\mathrm{O}_{2}(\mathrm{~g}) \longrightarrow 2 \mathrm{NO}_{2}(\mathrm{~g})\)
which of the following is correct?
320548
Assertion :
For the reaction,
\(2 \mathrm{~N}_{2} \mathrm{O}_{5} \rightarrow 4 \mathrm{NO}_{2}+\mathrm{O}_{2}\), Rate \(=\mathrm{k}\left[\mathrm{N}_{2} \mathrm{O}_{5}\right]\)
Reason :
Rate of decomposition of \(\mathrm{N}_{2} \mathrm{O}_{5}\) is determined by slow step.
320551
A certain reaction occurs in two steps as
(i) \({\rm{2S}}{{\rm{O}}_{\rm{2}}}{\rm{(g) + 2N}}{{\rm{O}}_{\rm{2}}}{\rm{(g)}} \to {\rm{2S}}{{\rm{O}}_{\rm{3}}}{\rm{(g) + }}\)
\(\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{2NO(g)}}\)
(ii) \(2 \mathrm{NO}(\mathrm{g})+\mathrm{O}_{2}(\mathrm{~g}) \longrightarrow 2 \mathrm{NO}_{2}(\mathrm{~g})\)
which of the following is correct?