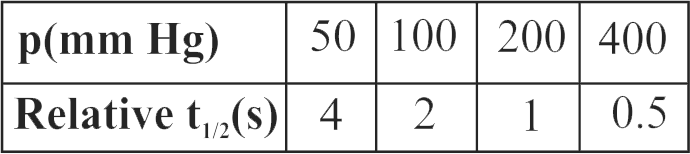
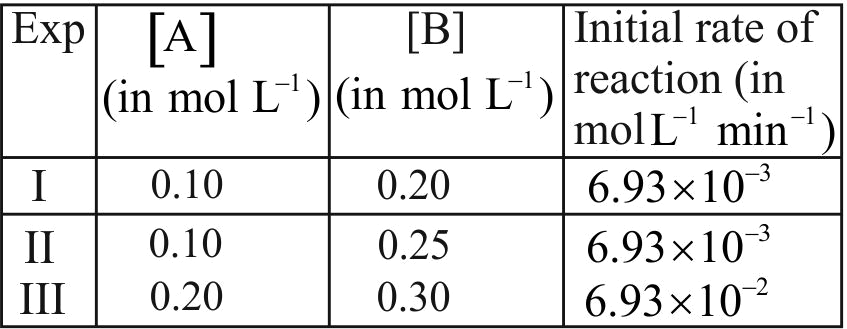
320506
The rates of a certain reaction \((\mathrm{dc} / \mathrm{dt})\) at different times are as follows?
\(\begin{array}{*{20}{c}}{{\rm{Time}}}&{{\rm{Rate}}\,{\rm{(mole}}\,\,{\rm{lite}}{{\rm{r}}^{{\rm{ - 1}}}}\,{\rm{se}}{{\rm{c}}^{{\rm{ - 1}}}}{\rm{)}}}\\{\rm{0}}&{{\rm{2}}{\rm{.8 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\\{{\rm{10}}}&{{\rm{2}}{\rm{.78 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\\{{\rm{20}}}&{{\rm{2}}{\rm{.81 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\\{{\rm{30}}}&{{\rm{2}}{\rm{.79 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\end{array}\)
The reaction is
320507 For the reaction \(\mathrm{aA} \rightarrow \mathrm{xP}\) when \({\text{[A] = 2}}{\text{.2 mM}}\) the rate was found to be \({\text{2}}{\text{.4 m M}}{{\text{s}}^{{\text{ - 1}}}}\). On reducing the concentration of A to half, the rate changes to \({\text{0}}{\text{.6 mM}}{{\text{s}}^{{\text{ - 1}}}}\). The order of reaction with respect to \(\mathrm{A}\) is
320506
The rates of a certain reaction \((\mathrm{dc} / \mathrm{dt})\) at different times are as follows?
\(\begin{array}{*{20}{c}}{{\rm{Time}}}&{{\rm{Rate}}\,{\rm{(mole}}\,\,{\rm{lite}}{{\rm{r}}^{{\rm{ - 1}}}}\,{\rm{se}}{{\rm{c}}^{{\rm{ - 1}}}}{\rm{)}}}\\{\rm{0}}&{{\rm{2}}{\rm{.8 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\\{{\rm{10}}}&{{\rm{2}}{\rm{.78 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\\{{\rm{20}}}&{{\rm{2}}{\rm{.81 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\\{{\rm{30}}}&{{\rm{2}}{\rm{.79 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\end{array}\)
The reaction is
320507 For the reaction \(\mathrm{aA} \rightarrow \mathrm{xP}\) when \({\text{[A] = 2}}{\text{.2 mM}}\) the rate was found to be \({\text{2}}{\text{.4 m M}}{{\text{s}}^{{\text{ - 1}}}}\). On reducing the concentration of A to half, the rate changes to \({\text{0}}{\text{.6 mM}}{{\text{s}}^{{\text{ - 1}}}}\). The order of reaction with respect to \(\mathrm{A}\) is
320506
The rates of a certain reaction \((\mathrm{dc} / \mathrm{dt})\) at different times are as follows?
\(\begin{array}{*{20}{c}}{{\rm{Time}}}&{{\rm{Rate}}\,{\rm{(mole}}\,\,{\rm{lite}}{{\rm{r}}^{{\rm{ - 1}}}}\,{\rm{se}}{{\rm{c}}^{{\rm{ - 1}}}}{\rm{)}}}\\{\rm{0}}&{{\rm{2}}{\rm{.8 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\\{{\rm{10}}}&{{\rm{2}}{\rm{.78 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\\{{\rm{20}}}&{{\rm{2}}{\rm{.81 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\\{{\rm{30}}}&{{\rm{2}}{\rm{.79 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\end{array}\)
The reaction is
320507 For the reaction \(\mathrm{aA} \rightarrow \mathrm{xP}\) when \({\text{[A] = 2}}{\text{.2 mM}}\) the rate was found to be \({\text{2}}{\text{.4 m M}}{{\text{s}}^{{\text{ - 1}}}}\). On reducing the concentration of A to half, the rate changes to \({\text{0}}{\text{.6 mM}}{{\text{s}}^{{\text{ - 1}}}}\). The order of reaction with respect to \(\mathrm{A}\) is
320506
The rates of a certain reaction \((\mathrm{dc} / \mathrm{dt})\) at different times are as follows?
\(\begin{array}{*{20}{c}}{{\rm{Time}}}&{{\rm{Rate}}\,{\rm{(mole}}\,\,{\rm{lite}}{{\rm{r}}^{{\rm{ - 1}}}}\,{\rm{se}}{{\rm{c}}^{{\rm{ - 1}}}}{\rm{)}}}\\{\rm{0}}&{{\rm{2}}{\rm{.8 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\\{{\rm{10}}}&{{\rm{2}}{\rm{.78 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\\{{\rm{20}}}&{{\rm{2}}{\rm{.81 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\\{{\rm{30}}}&{{\rm{2}}{\rm{.79 \times 1}}{{\rm{0}}^{{\rm{ - 2}}}}}\end{array}\)
The reaction is
320507 For the reaction \(\mathrm{aA} \rightarrow \mathrm{xP}\) when \({\text{[A] = 2}}{\text{.2 mM}}\) the rate was found to be \({\text{2}}{\text{.4 m M}}{{\text{s}}^{{\text{ - 1}}}}\). On reducing the concentration of A to half, the rate changes to \({\text{0}}{\text{.6 mM}}{{\text{s}}^{{\text{ - 1}}}}\). The order of reaction with respect to \(\mathrm{A}\) is