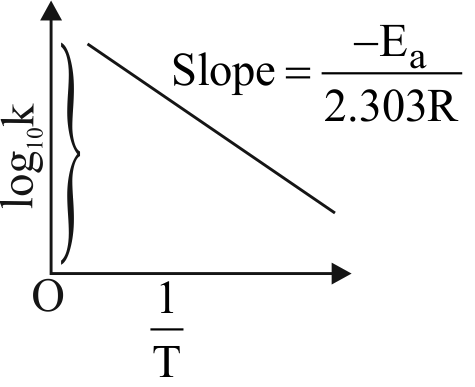
320222 The slope of Arrhenius Plot \(\left( {{\rm{ln}}\,{\rm{k}}\,{\rm{vs}}\frac{{\rm{1}}}{{\rm{T}}}} \right)\) of first order reaction is \(-5 \times 10^{3} \mathrm{~K}\). The value of \(\mathrm{E}_{\mathrm{a}}\) of the reaction is. Choose the correct option for your answer. [Given \(\mathrm{R}=8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1}\) ]
320224 An endothermic reaction \({\text{A}} \to {\text{B}}\) has \({{\rm{E}}_{\rm{a}}}\,\,{\rm{values}}\,\,{\rm{as}}\,\,{\rm{15}}{\mkern 1mu} {\mkern 1mu} {\rm{K}}{\rm{.}}{\mkern 1mu} {\mkern 1mu} {\rm{Cal/mol}}\) and enthalpy change is \({\rm{5}}\;{\rm{K}}{\rm{.Cal/mole}}\). The \({{\rm{E}}_{\rm{a}}}{\mkern 1mu} {\mkern 1mu} {\rm{of}}{\mkern 1mu} {\mkern 1mu} {\rm{B}}{\mkern 1mu} \to {\rm{A}}\) is ( in K.Cal/mole)
320222 The slope of Arrhenius Plot \(\left( {{\rm{ln}}\,{\rm{k}}\,{\rm{vs}}\frac{{\rm{1}}}{{\rm{T}}}} \right)\) of first order reaction is \(-5 \times 10^{3} \mathrm{~K}\). The value of \(\mathrm{E}_{\mathrm{a}}\) of the reaction is. Choose the correct option for your answer. [Given \(\mathrm{R}=8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1}\) ]
320224 An endothermic reaction \({\text{A}} \to {\text{B}}\) has \({{\rm{E}}_{\rm{a}}}\,\,{\rm{values}}\,\,{\rm{as}}\,\,{\rm{15}}{\mkern 1mu} {\mkern 1mu} {\rm{K}}{\rm{.}}{\mkern 1mu} {\mkern 1mu} {\rm{Cal/mol}}\) and enthalpy change is \({\rm{5}}\;{\rm{K}}{\rm{.Cal/mole}}\). The \({{\rm{E}}_{\rm{a}}}{\mkern 1mu} {\mkern 1mu} {\rm{of}}{\mkern 1mu} {\mkern 1mu} {\rm{B}}{\mkern 1mu} \to {\rm{A}}\) is ( in K.Cal/mole)
320222 The slope of Arrhenius Plot \(\left( {{\rm{ln}}\,{\rm{k}}\,{\rm{vs}}\frac{{\rm{1}}}{{\rm{T}}}} \right)\) of first order reaction is \(-5 \times 10^{3} \mathrm{~K}\). The value of \(\mathrm{E}_{\mathrm{a}}\) of the reaction is. Choose the correct option for your answer. [Given \(\mathrm{R}=8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1}\) ]
320224 An endothermic reaction \({\text{A}} \to {\text{B}}\) has \({{\rm{E}}_{\rm{a}}}\,\,{\rm{values}}\,\,{\rm{as}}\,\,{\rm{15}}{\mkern 1mu} {\mkern 1mu} {\rm{K}}{\rm{.}}{\mkern 1mu} {\mkern 1mu} {\rm{Cal/mol}}\) and enthalpy change is \({\rm{5}}\;{\rm{K}}{\rm{.Cal/mole}}\). The \({{\rm{E}}_{\rm{a}}}{\mkern 1mu} {\mkern 1mu} {\rm{of}}{\mkern 1mu} {\mkern 1mu} {\rm{B}}{\mkern 1mu} \to {\rm{A}}\) is ( in K.Cal/mole)
320222 The slope of Arrhenius Plot \(\left( {{\rm{ln}}\,{\rm{k}}\,{\rm{vs}}\frac{{\rm{1}}}{{\rm{T}}}} \right)\) of first order reaction is \(-5 \times 10^{3} \mathrm{~K}\). The value of \(\mathrm{E}_{\mathrm{a}}\) of the reaction is. Choose the correct option for your answer. [Given \(\mathrm{R}=8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1}\) ]
320224 An endothermic reaction \({\text{A}} \to {\text{B}}\) has \({{\rm{E}}_{\rm{a}}}\,\,{\rm{values}}\,\,{\rm{as}}\,\,{\rm{15}}{\mkern 1mu} {\mkern 1mu} {\rm{K}}{\rm{.}}{\mkern 1mu} {\mkern 1mu} {\rm{Cal/mol}}\) and enthalpy change is \({\rm{5}}\;{\rm{K}}{\rm{.Cal/mole}}\). The \({{\rm{E}}_{\rm{a}}}{\mkern 1mu} {\mkern 1mu} {\rm{of}}{\mkern 1mu} {\mkern 1mu} {\rm{B}}{\mkern 1mu} \to {\rm{A}}\) is ( in K.Cal/mole)
320222 The slope of Arrhenius Plot \(\left( {{\rm{ln}}\,{\rm{k}}\,{\rm{vs}}\frac{{\rm{1}}}{{\rm{T}}}} \right)\) of first order reaction is \(-5 \times 10^{3} \mathrm{~K}\). The value of \(\mathrm{E}_{\mathrm{a}}\) of the reaction is. Choose the correct option for your answer. [Given \(\mathrm{R}=8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1}\) ]
320224 An endothermic reaction \({\text{A}} \to {\text{B}}\) has \({{\rm{E}}_{\rm{a}}}\,\,{\rm{values}}\,\,{\rm{as}}\,\,{\rm{15}}{\mkern 1mu} {\mkern 1mu} {\rm{K}}{\rm{.}}{\mkern 1mu} {\mkern 1mu} {\rm{Cal/mol}}\) and enthalpy change is \({\rm{5}}\;{\rm{K}}{\rm{.Cal/mole}}\). The \({{\rm{E}}_{\rm{a}}}{\mkern 1mu} {\mkern 1mu} {\rm{of}}{\mkern 1mu} {\mkern 1mu} {\rm{B}}{\mkern 1mu} \to {\rm{A}}\) is ( in K.Cal/mole)