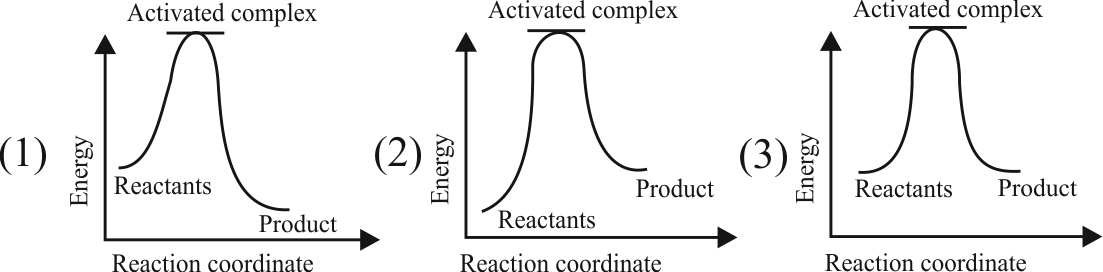
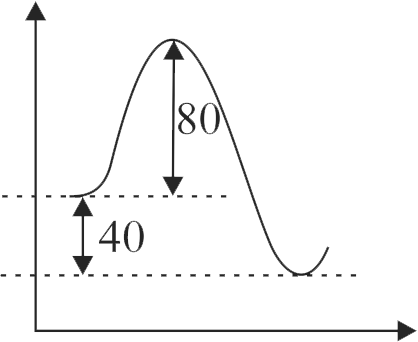
320109 For a given reaction, energy of activation for forward reaction \(\left(\mathrm{E}_{\mathrm{af}}\right)\) is \(80 \mathrm{~kJ} \mathrm{~mol}^{-1}\) and \(\Delta \mathrm{H}=40 \mathrm{~kJ} \mathrm{~mol}^{-1}\). A catalyst lowers \(\mathrm{E}_{\mathrm{af}}\) to \(20 \mathrm{~kJ} \mathrm{~mol}^{-1}\). The ratio of energy of activation for reverse reaction before and after addition of catalyst is
320109 For a given reaction, energy of activation for forward reaction \(\left(\mathrm{E}_{\mathrm{af}}\right)\) is \(80 \mathrm{~kJ} \mathrm{~mol}^{-1}\) and \(\Delta \mathrm{H}=40 \mathrm{~kJ} \mathrm{~mol}^{-1}\). A catalyst lowers \(\mathrm{E}_{\mathrm{af}}\) to \(20 \mathrm{~kJ} \mathrm{~mol}^{-1}\). The ratio of energy of activation for reverse reaction before and after addition of catalyst is
320109 For a given reaction, energy of activation for forward reaction \(\left(\mathrm{E}_{\mathrm{af}}\right)\) is \(80 \mathrm{~kJ} \mathrm{~mol}^{-1}\) and \(\Delta \mathrm{H}=40 \mathrm{~kJ} \mathrm{~mol}^{-1}\). A catalyst lowers \(\mathrm{E}_{\mathrm{af}}\) to \(20 \mathrm{~kJ} \mathrm{~mol}^{-1}\). The ratio of energy of activation for reverse reaction before and after addition of catalyst is
320109 For a given reaction, energy of activation for forward reaction \(\left(\mathrm{E}_{\mathrm{af}}\right)\) is \(80 \mathrm{~kJ} \mathrm{~mol}^{-1}\) and \(\Delta \mathrm{H}=40 \mathrm{~kJ} \mathrm{~mol}^{-1}\). A catalyst lowers \(\mathrm{E}_{\mathrm{af}}\) to \(20 \mathrm{~kJ} \mathrm{~mol}^{-1}\). The ratio of energy of activation for reverse reaction before and after addition of catalyst is