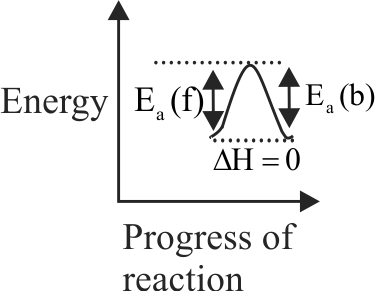
320098 \({\mathrm{\mathrm{A} \rightarrow \mathrm{B}, \Delta \mathrm{H}=-10 \mathrm{~kJ} \mathrm{~mol}^{-1}\, \mathrm{Ea}(\mathrm{f})=50 \mathrm{~kJ} \mathrm{~mol}^{-1}}}\)Then \({\mathrm{\mathrm{Ea}(\mathrm{b}) \mathrm{B} \rightarrow \mathrm{A}}}\) in j \({\rm{mo}}{{\rm{l}}^{ - 1}}\) will be?
320098 \({\mathrm{\mathrm{A} \rightarrow \mathrm{B}, \Delta \mathrm{H}=-10 \mathrm{~kJ} \mathrm{~mol}^{-1}\, \mathrm{Ea}(\mathrm{f})=50 \mathrm{~kJ} \mathrm{~mol}^{-1}}}\)Then \({\mathrm{\mathrm{Ea}(\mathrm{b}) \mathrm{B} \rightarrow \mathrm{A}}}\) in j \({\rm{mo}}{{\rm{l}}^{ - 1}}\) will be?
320098 \({\mathrm{\mathrm{A} \rightarrow \mathrm{B}, \Delta \mathrm{H}=-10 \mathrm{~kJ} \mathrm{~mol}^{-1}\, \mathrm{Ea}(\mathrm{f})=50 \mathrm{~kJ} \mathrm{~mol}^{-1}}}\)Then \({\mathrm{\mathrm{Ea}(\mathrm{b}) \mathrm{B} \rightarrow \mathrm{A}}}\) in j \({\rm{mo}}{{\rm{l}}^{ - 1}}\) will be?
320098 \({\mathrm{\mathrm{A} \rightarrow \mathrm{B}, \Delta \mathrm{H}=-10 \mathrm{~kJ} \mathrm{~mol}^{-1}\, \mathrm{Ea}(\mathrm{f})=50 \mathrm{~kJ} \mathrm{~mol}^{-1}}}\)Then \({\mathrm{\mathrm{Ea}(\mathrm{b}) \mathrm{B} \rightarrow \mathrm{A}}}\) in j \({\rm{mo}}{{\rm{l}}^{ - 1}}\) will be?