320093
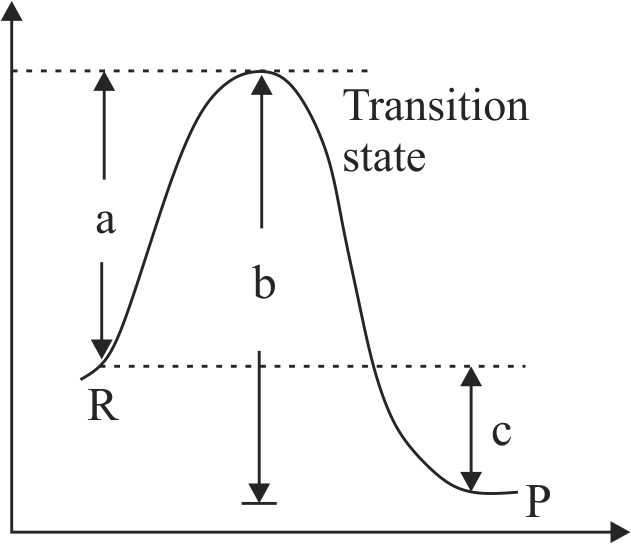
Assertion :
According to transition state theory, for the formation of an activated complex, one of the vibrational degree of freedom is converted into a translational degree of freedom.
Reason :
Energy of the activated complex is higher than energy of reactant molecules.
320095
Rate of a general reaction \(A+B \rightarrow\) products can be expressed as follows on the basis of collision theory. Rate \({\rm{ = }}{{\rm{Z}}_{{\rm{AB}}}}{{\rm{e}}^{{\rm{ - }}{{\rm{E}}_{\rm{a}}}{\rm{/RT}}}}\)
Which of the following statements is not correct for the above expression?
320096
Assertion :
According to transition state theory, for the formation of an activated complex, one of the vibrational degrees of freedom is converted into a translational degree of freedom.
Reason :
Energy of the activated complex is higher than the energy of reactant molecules.
320093
Assertion :
According to transition state theory, for the formation of an activated complex, one of the vibrational degree of freedom is converted into a translational degree of freedom.
Reason :
Energy of the activated complex is higher than energy of reactant molecules.
320095
Rate of a general reaction \(A+B \rightarrow\) products can be expressed as follows on the basis of collision theory. Rate \({\rm{ = }}{{\rm{Z}}_{{\rm{AB}}}}{{\rm{e}}^{{\rm{ - }}{{\rm{E}}_{\rm{a}}}{\rm{/RT}}}}\)
Which of the following statements is not correct for the above expression?
320096
Assertion :
According to transition state theory, for the formation of an activated complex, one of the vibrational degrees of freedom is converted into a translational degree of freedom.
Reason :
Energy of the activated complex is higher than the energy of reactant molecules.
320093
Assertion :
According to transition state theory, for the formation of an activated complex, one of the vibrational degree of freedom is converted into a translational degree of freedom.
Reason :
Energy of the activated complex is higher than energy of reactant molecules.
320095
Rate of a general reaction \(A+B \rightarrow\) products can be expressed as follows on the basis of collision theory. Rate \({\rm{ = }}{{\rm{Z}}_{{\rm{AB}}}}{{\rm{e}}^{{\rm{ - }}{{\rm{E}}_{\rm{a}}}{\rm{/RT}}}}\)
Which of the following statements is not correct for the above expression?
320096
Assertion :
According to transition state theory, for the formation of an activated complex, one of the vibrational degrees of freedom is converted into a translational degree of freedom.
Reason :
Energy of the activated complex is higher than the energy of reactant molecules.
320093
Assertion :
According to transition state theory, for the formation of an activated complex, one of the vibrational degree of freedom is converted into a translational degree of freedom.
Reason :
Energy of the activated complex is higher than energy of reactant molecules.
320095
Rate of a general reaction \(A+B \rightarrow\) products can be expressed as follows on the basis of collision theory. Rate \({\rm{ = }}{{\rm{Z}}_{{\rm{AB}}}}{{\rm{e}}^{{\rm{ - }}{{\rm{E}}_{\rm{a}}}{\rm{/RT}}}}\)
Which of the following statements is not correct for the above expression?
320096
Assertion :
According to transition state theory, for the formation of an activated complex, one of the vibrational degrees of freedom is converted into a translational degree of freedom.
Reason :
Energy of the activated complex is higher than the energy of reactant molecules.
320093
Assertion :
According to transition state theory, for the formation of an activated complex, one of the vibrational degree of freedom is converted into a translational degree of freedom.
Reason :
Energy of the activated complex is higher than energy of reactant molecules.
320095
Rate of a general reaction \(A+B \rightarrow\) products can be expressed as follows on the basis of collision theory. Rate \({\rm{ = }}{{\rm{Z}}_{{\rm{AB}}}}{{\rm{e}}^{{\rm{ - }}{{\rm{E}}_{\rm{a}}}{\rm{/RT}}}}\)
Which of the following statements is not correct for the above expression?
320096
Assertion :
According to transition state theory, for the formation of an activated complex, one of the vibrational degrees of freedom is converted into a translational degree of freedom.
Reason :
Energy of the activated complex is higher than the energy of reactant molecules.