330416
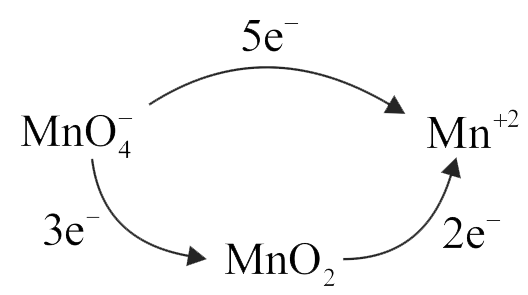
Given that, \(\mathrm{MnO}_{4}^{-}+8 \mathrm{H}^{+}+5 \mathrm{e}^{-} \rightarrow\)
\({\rm{M}}{{\rm{n}}^{2 + }} + 4{{\rm{H}}_{\rm{2}}}{\rm{O}}\,;{\rm{E}}^\circ = 1.51\;{\rm{V}}\)
\({\rm{Mn}}{{\rm{O}}_2} + 4{{\rm{H}}^ + } + 2{{\rm{e}}^ - } \to \)
\({\rm{M}}{{\rm{n}}^{2 + }} + 2{{\rm{H}}_{\rm{2}}}{\rm{O}}\,;{\rm{E}}^\circ = 1.23\;{\rm{V}}\)
\({{\rm{E}}_{{\rm{MnO}}_4^ - /{\rm{Mn}}{{\rm{O}}_2}}}\) is
330417
For a \(\mathrm{Ag}-\mathrm{Zn}\) button cell, net reaction is
\(\mathrm{Zn}(\mathrm{s})+\mathrm{Ag}_{2} \mathrm{O}(\mathrm{s}) \rightarrow \mathrm{ZnO}(\mathrm{s})+2 \mathrm{Ag}(\mathrm{s})\)
\(\Delta {\text{G}}_{\text{f}}^0\left( {{\text{A}}{{\text{g}}_2}{\text{O}}} \right) = - 11.21\,\,{\text{kJ mo}}{{\text{l}}^{ - 1}}\)
\(\Delta {\text{G}}_{\text{f}}^0({\text{ZnO}}) = - 318.3\,\,{\text{kJ mo}}{{\text{l}}^{ - 1}}\)
Then, \(\mathrm{E}_{\text {cell }}^{0}\) of the button cell is
330419
Given:
\({\rm{(i)}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}{\rm{ + 2}}{{\rm{e}}^{\rm{ - }}} \to {\rm{Cu,}}{{\rm{E}}^{\rm{o}}}{\rm{ = 0}}{\rm{.337V}}\)
\({\rm{(ii)}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}{\rm{ + e}} \to {\rm{C}}{{\rm{u}}^{\rm{ + }}}{\rm{,}}{{\rm{E}}^{\rm{o}}}{\rm{ = 0}}{\rm{.153V}}\)
Electrode potential, \({E^o}\) for the reaction,
\(C{u^ + } + {e^ - } \to Cu\), will be
330416
Given that, \(\mathrm{MnO}_{4}^{-}+8 \mathrm{H}^{+}+5 \mathrm{e}^{-} \rightarrow\)
\({\rm{M}}{{\rm{n}}^{2 + }} + 4{{\rm{H}}_{\rm{2}}}{\rm{O}}\,;{\rm{E}}^\circ = 1.51\;{\rm{V}}\)
\({\rm{Mn}}{{\rm{O}}_2} + 4{{\rm{H}}^ + } + 2{{\rm{e}}^ - } \to \)
\({\rm{M}}{{\rm{n}}^{2 + }} + 2{{\rm{H}}_{\rm{2}}}{\rm{O}}\,;{\rm{E}}^\circ = 1.23\;{\rm{V}}\)
\({{\rm{E}}_{{\rm{MnO}}_4^ - /{\rm{Mn}}{{\rm{O}}_2}}}\) is
330417
For a \(\mathrm{Ag}-\mathrm{Zn}\) button cell, net reaction is
\(\mathrm{Zn}(\mathrm{s})+\mathrm{Ag}_{2} \mathrm{O}(\mathrm{s}) \rightarrow \mathrm{ZnO}(\mathrm{s})+2 \mathrm{Ag}(\mathrm{s})\)
\(\Delta {\text{G}}_{\text{f}}^0\left( {{\text{A}}{{\text{g}}_2}{\text{O}}} \right) = - 11.21\,\,{\text{kJ mo}}{{\text{l}}^{ - 1}}\)
\(\Delta {\text{G}}_{\text{f}}^0({\text{ZnO}}) = - 318.3\,\,{\text{kJ mo}}{{\text{l}}^{ - 1}}\)
Then, \(\mathrm{E}_{\text {cell }}^{0}\) of the button cell is
330419
Given:
\({\rm{(i)}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}{\rm{ + 2}}{{\rm{e}}^{\rm{ - }}} \to {\rm{Cu,}}{{\rm{E}}^{\rm{o}}}{\rm{ = 0}}{\rm{.337V}}\)
\({\rm{(ii)}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}{\rm{ + e}} \to {\rm{C}}{{\rm{u}}^{\rm{ + }}}{\rm{,}}{{\rm{E}}^{\rm{o}}}{\rm{ = 0}}{\rm{.153V}}\)
Electrode potential, \({E^o}\) for the reaction,
\(C{u^ + } + {e^ - } \to Cu\), will be
330416
Given that, \(\mathrm{MnO}_{4}^{-}+8 \mathrm{H}^{+}+5 \mathrm{e}^{-} \rightarrow\)
\({\rm{M}}{{\rm{n}}^{2 + }} + 4{{\rm{H}}_{\rm{2}}}{\rm{O}}\,;{\rm{E}}^\circ = 1.51\;{\rm{V}}\)
\({\rm{Mn}}{{\rm{O}}_2} + 4{{\rm{H}}^ + } + 2{{\rm{e}}^ - } \to \)
\({\rm{M}}{{\rm{n}}^{2 + }} + 2{{\rm{H}}_{\rm{2}}}{\rm{O}}\,;{\rm{E}}^\circ = 1.23\;{\rm{V}}\)
\({{\rm{E}}_{{\rm{MnO}}_4^ - /{\rm{Mn}}{{\rm{O}}_2}}}\) is
330417
For a \(\mathrm{Ag}-\mathrm{Zn}\) button cell, net reaction is
\(\mathrm{Zn}(\mathrm{s})+\mathrm{Ag}_{2} \mathrm{O}(\mathrm{s}) \rightarrow \mathrm{ZnO}(\mathrm{s})+2 \mathrm{Ag}(\mathrm{s})\)
\(\Delta {\text{G}}_{\text{f}}^0\left( {{\text{A}}{{\text{g}}_2}{\text{O}}} \right) = - 11.21\,\,{\text{kJ mo}}{{\text{l}}^{ - 1}}\)
\(\Delta {\text{G}}_{\text{f}}^0({\text{ZnO}}) = - 318.3\,\,{\text{kJ mo}}{{\text{l}}^{ - 1}}\)
Then, \(\mathrm{E}_{\text {cell }}^{0}\) of the button cell is
330419
Given:
\({\rm{(i)}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}{\rm{ + 2}}{{\rm{e}}^{\rm{ - }}} \to {\rm{Cu,}}{{\rm{E}}^{\rm{o}}}{\rm{ = 0}}{\rm{.337V}}\)
\({\rm{(ii)}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}{\rm{ + e}} \to {\rm{C}}{{\rm{u}}^{\rm{ + }}}{\rm{,}}{{\rm{E}}^{\rm{o}}}{\rm{ = 0}}{\rm{.153V}}\)
Electrode potential, \({E^o}\) for the reaction,
\(C{u^ + } + {e^ - } \to Cu\), will be
330416
Given that, \(\mathrm{MnO}_{4}^{-}+8 \mathrm{H}^{+}+5 \mathrm{e}^{-} \rightarrow\)
\({\rm{M}}{{\rm{n}}^{2 + }} + 4{{\rm{H}}_{\rm{2}}}{\rm{O}}\,;{\rm{E}}^\circ = 1.51\;{\rm{V}}\)
\({\rm{Mn}}{{\rm{O}}_2} + 4{{\rm{H}}^ + } + 2{{\rm{e}}^ - } \to \)
\({\rm{M}}{{\rm{n}}^{2 + }} + 2{{\rm{H}}_{\rm{2}}}{\rm{O}}\,;{\rm{E}}^\circ = 1.23\;{\rm{V}}\)
\({{\rm{E}}_{{\rm{MnO}}_4^ - /{\rm{Mn}}{{\rm{O}}_2}}}\) is
330417
For a \(\mathrm{Ag}-\mathrm{Zn}\) button cell, net reaction is
\(\mathrm{Zn}(\mathrm{s})+\mathrm{Ag}_{2} \mathrm{O}(\mathrm{s}) \rightarrow \mathrm{ZnO}(\mathrm{s})+2 \mathrm{Ag}(\mathrm{s})\)
\(\Delta {\text{G}}_{\text{f}}^0\left( {{\text{A}}{{\text{g}}_2}{\text{O}}} \right) = - 11.21\,\,{\text{kJ mo}}{{\text{l}}^{ - 1}}\)
\(\Delta {\text{G}}_{\text{f}}^0({\text{ZnO}}) = - 318.3\,\,{\text{kJ mo}}{{\text{l}}^{ - 1}}\)
Then, \(\mathrm{E}_{\text {cell }}^{0}\) of the button cell is
330419
Given:
\({\rm{(i)}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}{\rm{ + 2}}{{\rm{e}}^{\rm{ - }}} \to {\rm{Cu,}}{{\rm{E}}^{\rm{o}}}{\rm{ = 0}}{\rm{.337V}}\)
\({\rm{(ii)}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}{\rm{ + e}} \to {\rm{C}}{{\rm{u}}^{\rm{ + }}}{\rm{,}}{{\rm{E}}^{\rm{o}}}{\rm{ = 0}}{\rm{.153V}}\)
Electrode potential, \({E^o}\) for the reaction,
\(C{u^ + } + {e^ - } \to Cu\), will be