330058
Given below are half cell reactions :
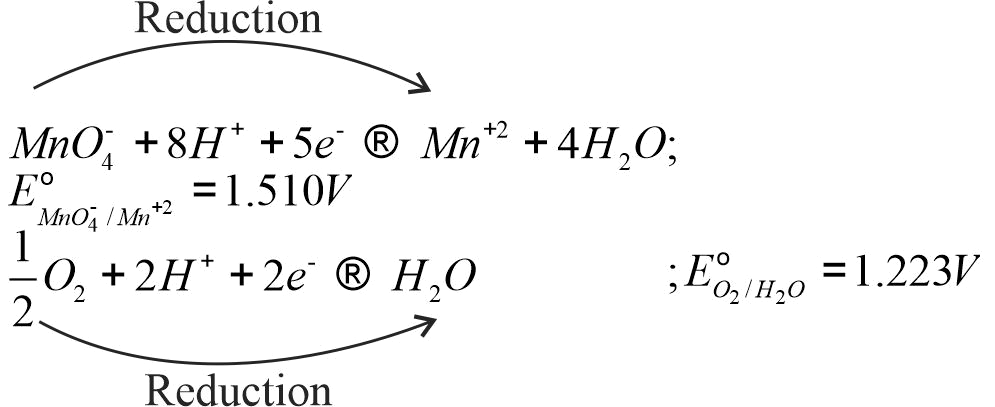
\(MnO_4^ - + 8{H^ + }5{e^ - } \to M{n^{2 + }} + 4{H_2}O,\)
\({\rm{E}}_{{\rm{M}}{{\rm{n}}^{{\rm{2 + }}}}{\rm{/MnO}}_{\rm{4}}^{\rm{ - }}}^{\rm{o}}{\rm{ = - 1}}{\rm{.510V}}\)
\(\frac{1}{2}{O_2} + 2{H^ + } + 2{e^ - } \to {H_2}O\)
\({\rm{E}}_{{{\rm{O}}_{\rm{2}}}{\rm{/}}{{\rm{H}}_{\rm{2}}}{\rm{O}}}^{\rm{o}}{\rm{ = + 1}}{\rm{.223V}}\)
Will the permanganate ion, \({\rm{MnO}}_{\rm{4}}^{\rm{ - }}\,\,{\rm{liberate}}\,\,{{\rm{O}}_{\rm{2}}}\) from water in the presence of an acid ?
330061
I.
The potential of individual half-cell can be measured.
II.
Difference between the potentials of two half-cells can only be measured.
III.
\(\left. {{\rm{Pt(s)}}} \right \vert \left. {{{\rm{H}}_{\rm{2}}}{\rm{(g)}}} \right \vert {{\rm{H}}^{\rm{ + }}}{\rm{(aq)}}\) half-cell is called standard hydrogen electrode.
Select the correct statement(s) and choose the appropriate option.
330058
Given below are half cell reactions :
\(MnO_4^ - + 8{H^ + }5{e^ - } \to M{n^{2 + }} + 4{H_2}O,\)
\({\rm{E}}_{{\rm{M}}{{\rm{n}}^{{\rm{2 + }}}}{\rm{/MnO}}_{\rm{4}}^{\rm{ - }}}^{\rm{o}}{\rm{ = - 1}}{\rm{.510V}}\)
\(\frac{1}{2}{O_2} + 2{H^ + } + 2{e^ - } \to {H_2}O\)
\({\rm{E}}_{{{\rm{O}}_{\rm{2}}}{\rm{/}}{{\rm{H}}_{\rm{2}}}{\rm{O}}}^{\rm{o}}{\rm{ = + 1}}{\rm{.223V}}\)
Will the permanganate ion, \({\rm{MnO}}_{\rm{4}}^{\rm{ - }}\,\,{\rm{liberate}}\,\,{{\rm{O}}_{\rm{2}}}\) from water in the presence of an acid ?
330061
I.
The potential of individual half-cell can be measured.
II.
Difference between the potentials of two half-cells can only be measured.
III.
\(\left. {{\rm{Pt(s)}}} \right \vert \left. {{{\rm{H}}_{\rm{2}}}{\rm{(g)}}} \right \vert {{\rm{H}}^{\rm{ + }}}{\rm{(aq)}}\) half-cell is called standard hydrogen electrode.
Select the correct statement(s) and choose the appropriate option.
330058
Given below are half cell reactions :
\(MnO_4^ - + 8{H^ + }5{e^ - } \to M{n^{2 + }} + 4{H_2}O,\)
\({\rm{E}}_{{\rm{M}}{{\rm{n}}^{{\rm{2 + }}}}{\rm{/MnO}}_{\rm{4}}^{\rm{ - }}}^{\rm{o}}{\rm{ = - 1}}{\rm{.510V}}\)
\(\frac{1}{2}{O_2} + 2{H^ + } + 2{e^ - } \to {H_2}O\)
\({\rm{E}}_{{{\rm{O}}_{\rm{2}}}{\rm{/}}{{\rm{H}}_{\rm{2}}}{\rm{O}}}^{\rm{o}}{\rm{ = + 1}}{\rm{.223V}}\)
Will the permanganate ion, \({\rm{MnO}}_{\rm{4}}^{\rm{ - }}\,\,{\rm{liberate}}\,\,{{\rm{O}}_{\rm{2}}}\) from water in the presence of an acid ?
330061
I.
The potential of individual half-cell can be measured.
II.
Difference between the potentials of two half-cells can only be measured.
III.
\(\left. {{\rm{Pt(s)}}} \right \vert \left. {{{\rm{H}}_{\rm{2}}}{\rm{(g)}}} \right \vert {{\rm{H}}^{\rm{ + }}}{\rm{(aq)}}\) half-cell is called standard hydrogen electrode.
Select the correct statement(s) and choose the appropriate option.
330058
Given below are half cell reactions :
\(MnO_4^ - + 8{H^ + }5{e^ - } \to M{n^{2 + }} + 4{H_2}O,\)
\({\rm{E}}_{{\rm{M}}{{\rm{n}}^{{\rm{2 + }}}}{\rm{/MnO}}_{\rm{4}}^{\rm{ - }}}^{\rm{o}}{\rm{ = - 1}}{\rm{.510V}}\)
\(\frac{1}{2}{O_2} + 2{H^ + } + 2{e^ - } \to {H_2}O\)
\({\rm{E}}_{{{\rm{O}}_{\rm{2}}}{\rm{/}}{{\rm{H}}_{\rm{2}}}{\rm{O}}}^{\rm{o}}{\rm{ = + 1}}{\rm{.223V}}\)
Will the permanganate ion, \({\rm{MnO}}_{\rm{4}}^{\rm{ - }}\,\,{\rm{liberate}}\,\,{{\rm{O}}_{\rm{2}}}\) from water in the presence of an acid ?
330061
I.
The potential of individual half-cell can be measured.
II.
Difference between the potentials of two half-cells can only be measured.
III.
\(\left. {{\rm{Pt(s)}}} \right \vert \left. {{{\rm{H}}_{\rm{2}}}{\rm{(g)}}} \right \vert {{\rm{H}}^{\rm{ + }}}{\rm{(aq)}}\) half-cell is called standard hydrogen electrode.
Select the correct statement(s) and choose the appropriate option.