319434
Assertion :
The pressure exerted by the vapour over the surface of the liquid at the equilibrium at a given temperature is called the vapour pressure of the liquid.
Reason :
If a non-volatile solute is added to a solvent to give a solution, the vapour pressure of the solution is found to be greater than the vapour pressure of the pure solvent.
319435
Two beakers of capacity \(500 \mathrm{~mL}\) were taken. One of these beakers, labelled as " \({\text{A}}\) ", was filled with \(400 \mathrm{~mL}\) water whereas the beaker labelled " \({\text{B}}\) " was filled with \(400 \mathrm{~mL}\) of \({\text{2}}\,\,{\text{M}}\) solution of \(\mathrm{NaCl}\). At the same temperature both the beakers were placed in closed containers of same material and same capactiy as shown in figure: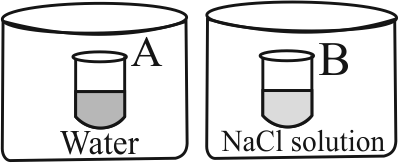
At a given temperature , which of the following statement is correct about the vapour pressure of pure water and that of NaCl solution?
319434
Assertion :
The pressure exerted by the vapour over the surface of the liquid at the equilibrium at a given temperature is called the vapour pressure of the liquid.
Reason :
If a non-volatile solute is added to a solvent to give a solution, the vapour pressure of the solution is found to be greater than the vapour pressure of the pure solvent.
319435
Two beakers of capacity \(500 \mathrm{~mL}\) were taken. One of these beakers, labelled as " \({\text{A}}\) ", was filled with \(400 \mathrm{~mL}\) water whereas the beaker labelled " \({\text{B}}\) " was filled with \(400 \mathrm{~mL}\) of \({\text{2}}\,\,{\text{M}}\) solution of \(\mathrm{NaCl}\). At the same temperature both the beakers were placed in closed containers of same material and same capactiy as shown in figure:
At a given temperature , which of the following statement is correct about the vapour pressure of pure water and that of NaCl solution?
319434
Assertion :
The pressure exerted by the vapour over the surface of the liquid at the equilibrium at a given temperature is called the vapour pressure of the liquid.
Reason :
If a non-volatile solute is added to a solvent to give a solution, the vapour pressure of the solution is found to be greater than the vapour pressure of the pure solvent.
319435
Two beakers of capacity \(500 \mathrm{~mL}\) were taken. One of these beakers, labelled as " \({\text{A}}\) ", was filled with \(400 \mathrm{~mL}\) water whereas the beaker labelled " \({\text{B}}\) " was filled with \(400 \mathrm{~mL}\) of \({\text{2}}\,\,{\text{M}}\) solution of \(\mathrm{NaCl}\). At the same temperature both the beakers were placed in closed containers of same material and same capactiy as shown in figure:
At a given temperature , which of the following statement is correct about the vapour pressure of pure water and that of NaCl solution?
319434
Assertion :
The pressure exerted by the vapour over the surface of the liquid at the equilibrium at a given temperature is called the vapour pressure of the liquid.
Reason :
If a non-volatile solute is added to a solvent to give a solution, the vapour pressure of the solution is found to be greater than the vapour pressure of the pure solvent.
319435
Two beakers of capacity \(500 \mathrm{~mL}\) were taken. One of these beakers, labelled as " \({\text{A}}\) ", was filled with \(400 \mathrm{~mL}\) water whereas the beaker labelled " \({\text{B}}\) " was filled with \(400 \mathrm{~mL}\) of \({\text{2}}\,\,{\text{M}}\) solution of \(\mathrm{NaCl}\). At the same temperature both the beakers were placed in closed containers of same material and same capactiy as shown in figure:
At a given temperature , which of the following statement is correct about the vapour pressure of pure water and that of NaCl solution?