319434
Assertion :
The pressure exerted by the vapour over the surface of the liquid at the equilibrium at a given temperature is called the vapour pressure of the liquid.
Reason :
If a non-volatile solute is added to a solvent to give a solution, the vapour pressure of the solution is found to be greater than the vapour pressure of the pure solvent.
319435
Two beakers of capacity 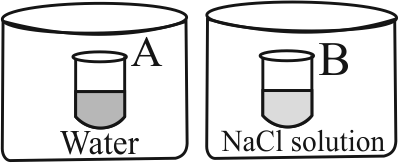
At a given temperature , which of the following statement is correct about the vapour pressure of pure water and that of NaCl solution?
319434
Assertion :
The pressure exerted by the vapour over the surface of the liquid at the equilibrium at a given temperature is called the vapour pressure of the liquid.
Reason :
If a non-volatile solute is added to a solvent to give a solution, the vapour pressure of the solution is found to be greater than the vapour pressure of the pure solvent.
319435
Two beakers of capacity 
At a given temperature , which of the following statement is correct about the vapour pressure of pure water and that of NaCl solution?
319434
Assertion :
The pressure exerted by the vapour over the surface of the liquid at the equilibrium at a given temperature is called the vapour pressure of the liquid.
Reason :
If a non-volatile solute is added to a solvent to give a solution, the vapour pressure of the solution is found to be greater than the vapour pressure of the pure solvent.
319435
Two beakers of capacity 
At a given temperature , which of the following statement is correct about the vapour pressure of pure water and that of NaCl solution?
319434
Assertion :
The pressure exerted by the vapour over the surface of the liquid at the equilibrium at a given temperature is called the vapour pressure of the liquid.
Reason :
If a non-volatile solute is added to a solvent to give a solution, the vapour pressure of the solution is found to be greater than the vapour pressure of the pure solvent.
319435
Two beakers of capacity 
At a given temperature , which of the following statement is correct about the vapour pressure of pure water and that of NaCl solution?
