319478
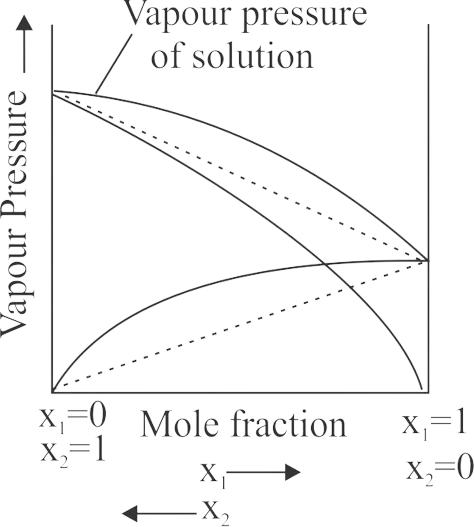
Assertion :
The mixing of two completely miscible liquid, A and B showing positive deviation from Raoult’s law is followed by an absorption of heat
Reason :
The A-B molecular interaction forces are stronger than the \({\rm{A - A}}\,\,{\rm{or}}\,\,{\rm{B - B}}\) molecular interaction forces.
319478
Assertion :
The mixing of two completely miscible liquid, A and B showing positive deviation from Raoult’s law is followed by an absorption of heat
Reason :
The A-B molecular interaction forces are stronger than the \({\rm{A - A}}\,\,{\rm{or}}\,\,{\rm{B - B}}\) molecular interaction forces.
319478
Assertion :
The mixing of two completely miscible liquid, A and B showing positive deviation from Raoult’s law is followed by an absorption of heat
Reason :
The A-B molecular interaction forces are stronger than the \({\rm{A - A}}\,\,{\rm{or}}\,\,{\rm{B - B}}\) molecular interaction forces.
319478
Assertion :
The mixing of two completely miscible liquid, A and B showing positive deviation from Raoult’s law is followed by an absorption of heat
Reason :
The A-B molecular interaction forces are stronger than the \({\rm{A - A}}\,\,{\rm{or}}\,\,{\rm{B - B}}\) molecular interaction forces.
319478
Assertion :
The mixing of two completely miscible liquid, A and B showing positive deviation from Raoult’s law is followed by an absorption of heat
Reason :
The A-B molecular interaction forces are stronger than the \({\rm{A - A}}\,\,{\rm{or}}\,\,{\rm{B - B}}\) molecular interaction forces.