319339
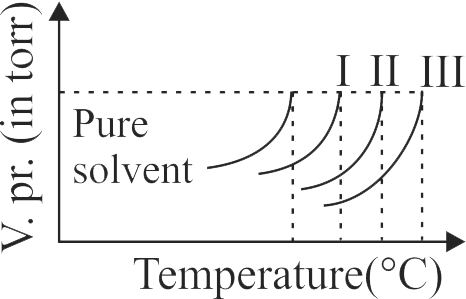
At a given temperature,
I. Vapour pressure of a solution containing non- volatile solute is proportional to mole fraction of solvent
II. Lowering of vapour pressure of solution containing non volatile solute is proportional to mole fraction of solute
III. Relative lowering of vapour pressure is equal to mole fraction of solute. The correct combination is
319339
At a given temperature,
I. Vapour pressure of a solution containing non- volatile solute is proportional to mole fraction of solvent
II. Lowering of vapour pressure of solution containing non volatile solute is proportional to mole fraction of solute
III. Relative lowering of vapour pressure is equal to mole fraction of solute. The correct combination is
319339
At a given temperature,
I. Vapour pressure of a solution containing non- volatile solute is proportional to mole fraction of solvent
II. Lowering of vapour pressure of solution containing non volatile solute is proportional to mole fraction of solute
III. Relative lowering of vapour pressure is equal to mole fraction of solute. The correct combination is
319339
At a given temperature,
I. Vapour pressure of a solution containing non- volatile solute is proportional to mole fraction of solvent
II. Lowering of vapour pressure of solution containing non volatile solute is proportional to mole fraction of solute
III. Relative lowering of vapour pressure is equal to mole fraction of solute. The correct combination is