318215
Statement A :
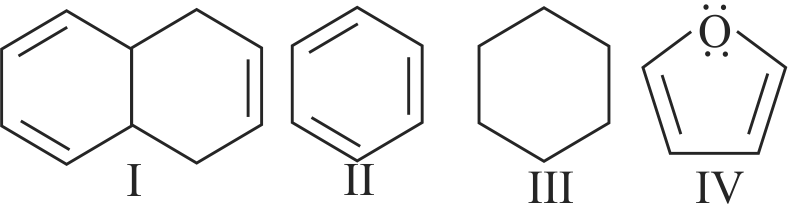
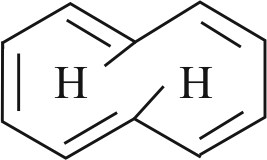
The compound cyclooctatetraene has the following structural formula.
It is cyclic and conjugated \(8\pi \)- electron system but it is not an aromatic compound.
Statement B :
\(\left( {{\text{4n + 2}}} \right){\text{ }}\pi {\text{ - }}\) electron rule does not hold good and ring is not planar.
318215
Statement A :
The compound cyclooctatetraene has the following structural formula.
It is cyclic and conjugated \(8\pi \)- electron system but it is not an aromatic compound.
Statement B :
\(\left( {{\text{4n + 2}}} \right){\text{ }}\pi {\text{ - }}\) electron rule does not hold good and ring is not planar.
318215
Statement A :
The compound cyclooctatetraene has the following structural formula.
It is cyclic and conjugated \(8\pi \)- electron system but it is not an aromatic compound.
Statement B :
\(\left( {{\text{4n + 2}}} \right){\text{ }}\pi {\text{ - }}\) electron rule does not hold good and ring is not planar.
318215
Statement A :
The compound cyclooctatetraene has the following structural formula.
It is cyclic and conjugated \(8\pi \)- electron system but it is not an aromatic compound.
Statement B :
\(\left( {{\text{4n + 2}}} \right){\text{ }}\pi {\text{ - }}\) electron rule does not hold good and ring is not planar.