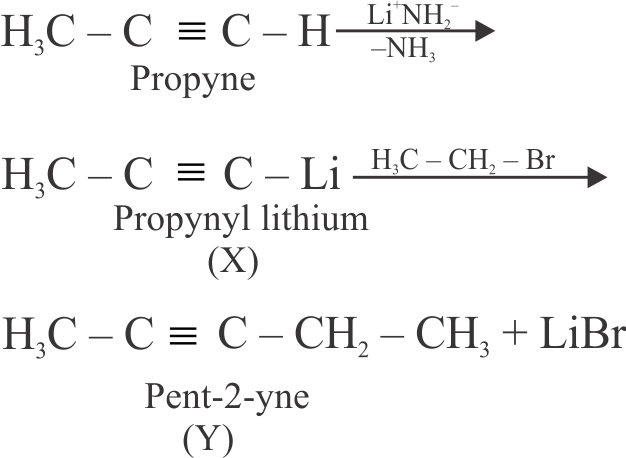
318160
Amongst the following, the total number of compounds which will undergo addition reaction will be:
(A) \({\mathrm{\mathrm{CH}_{3}-\mathrm{CH}=\mathrm{CH}_{2}}}\)
(B)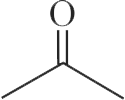
(C) \({\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}} \equiv {\text{N}}\)
(D)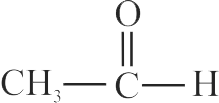
(E) \({\mathrm{\mathrm{CH}_{4}}}\)
(F) \({\mathrm{\mathrm{CH}_{3} \mathrm{C} \equiv \mathrm{CCH}_{3}}}\)
318160
Amongst the following, the total number of compounds which will undergo addition reaction will be:
(A) \({\mathrm{\mathrm{CH}_{3}-\mathrm{CH}=\mathrm{CH}_{2}}}\)
(B)
(C) \({\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}} \equiv {\text{N}}\)
(D)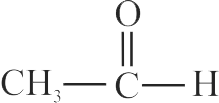
(E) \({\mathrm{\mathrm{CH}_{4}}}\)
(F) \({\mathrm{\mathrm{CH}_{3} \mathrm{C} \equiv \mathrm{CCH}_{3}}}\)
318160
Amongst the following, the total number of compounds which will undergo addition reaction will be:
(A) \({\mathrm{\mathrm{CH}_{3}-\mathrm{CH}=\mathrm{CH}_{2}}}\)
(B)
(C) \({\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}} \equiv {\text{N}}\)
(D)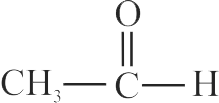
(E) \({\mathrm{\mathrm{CH}_{4}}}\)
(F) \({\mathrm{\mathrm{CH}_{3} \mathrm{C} \equiv \mathrm{CCH}_{3}}}\)
318160
Amongst the following, the total number of compounds which will undergo addition reaction will be:
(A) \({\mathrm{\mathrm{CH}_{3}-\mathrm{CH}=\mathrm{CH}_{2}}}\)
(B)
(C) \({\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}} \equiv {\text{N}}\)
(D)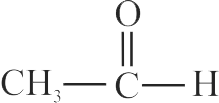
(E) \({\mathrm{\mathrm{CH}_{4}}}\)
(F) \({\mathrm{\mathrm{CH}_{3} \mathrm{C} \equiv \mathrm{CCH}_{3}}}\)