CHXI13:HYDROCARBONS
318104
Assertion :
The presence of enhances the solubility of alkenes in water.
Reason :
Alkenes are weakly polar in nature.
1 Both Assertion and Reason are correct and Reason is the correct explanation of the Assertion.
2 Both Assertion and Reason are correct but Reason is not the correct explanation of the Assertion.
3 Assertion is correct but Reason is incorrect.
4 Assertion is incorrect but Reason is correct.
Explanation:
forms complex with the alkene by bonding giving an ion and, thus the solubility increases.
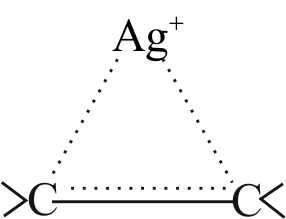
So, option (2) is correct