318066
In the following sequence of reactions, the compound ' \(\mathrm{A}\) ' is
\({\text{A}}\xrightarrow{{{\text{HBr}}}}{\text{B}}\xrightarrow{{{\text{Alc}}{\text{.KOH}}}}{\text{C}}\xrightarrow{{{{\text{O}}_{\text{3}}}{\text{,Zn/}}{{\text{H}}_{\text{2}}}{\text{O}}}}\)
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO + HCHO}}\)
318067
\(\mathop {{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}{\text{Cl}}}\limits_{\left[ {\text{A}} \right]} \xrightarrow[{{\text{KOH}}}]{{{\text{alc}}}}\left[ {\text{B}} \right]\xrightarrow{{{\text{HCl}}}}\left[ {\text{C}} \right]\xrightarrow[{{\text{KOH}}}]{{{\text{(aq)}}}}\mathop {{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}{\text{O}}}\limits_{\left[ {\text{D}} \right]} \)
The compound " A " is
318066
In the following sequence of reactions, the compound ' \(\mathrm{A}\) ' is
\({\text{A}}\xrightarrow{{{\text{HBr}}}}{\text{B}}\xrightarrow{{{\text{Alc}}{\text{.KOH}}}}{\text{C}}\xrightarrow{{{{\text{O}}_{\text{3}}}{\text{,Zn/}}{{\text{H}}_{\text{2}}}{\text{O}}}}\)
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO + HCHO}}\)
318067
\(\mathop {{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}{\text{Cl}}}\limits_{\left[ {\text{A}} \right]} \xrightarrow[{{\text{KOH}}}]{{{\text{alc}}}}\left[ {\text{B}} \right]\xrightarrow{{{\text{HCl}}}}\left[ {\text{C}} \right]\xrightarrow[{{\text{KOH}}}]{{{\text{(aq)}}}}\mathop {{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}{\text{O}}}\limits_{\left[ {\text{D}} \right]} \)
The compound " A " is
318066
In the following sequence of reactions, the compound ' \(\mathrm{A}\) ' is
\({\text{A}}\xrightarrow{{{\text{HBr}}}}{\text{B}}\xrightarrow{{{\text{Alc}}{\text{.KOH}}}}{\text{C}}\xrightarrow{{{{\text{O}}_{\text{3}}}{\text{,Zn/}}{{\text{H}}_{\text{2}}}{\text{O}}}}\)
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO + HCHO}}\)
318067
\(\mathop {{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}{\text{Cl}}}\limits_{\left[ {\text{A}} \right]} \xrightarrow[{{\text{KOH}}}]{{{\text{alc}}}}\left[ {\text{B}} \right]\xrightarrow{{{\text{HCl}}}}\left[ {\text{C}} \right]\xrightarrow[{{\text{KOH}}}]{{{\text{(aq)}}}}\mathop {{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}{\text{O}}}\limits_{\left[ {\text{D}} \right]} \)
The compound " A " is
318066
In the following sequence of reactions, the compound ' \(\mathrm{A}\) ' is
\({\text{A}}\xrightarrow{{{\text{HBr}}}}{\text{B}}\xrightarrow{{{\text{Alc}}{\text{.KOH}}}}{\text{C}}\xrightarrow{{{{\text{O}}_{\text{3}}}{\text{,Zn/}}{{\text{H}}_{\text{2}}}{\text{O}}}}\)
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO + HCHO}}\)
318067
\(\mathop {{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}{\text{Cl}}}\limits_{\left[ {\text{A}} \right]} \xrightarrow[{{\text{KOH}}}]{{{\text{alc}}}}\left[ {\text{B}} \right]\xrightarrow{{{\text{HCl}}}}\left[ {\text{C}} \right]\xrightarrow[{{\text{KOH}}}]{{{\text{(aq)}}}}\mathop {{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}{\text{O}}}\limits_{\left[ {\text{D}} \right]} \)
The compound " A " is
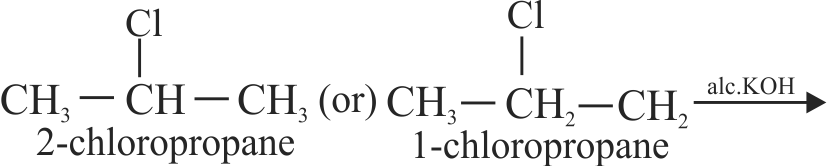
.png)