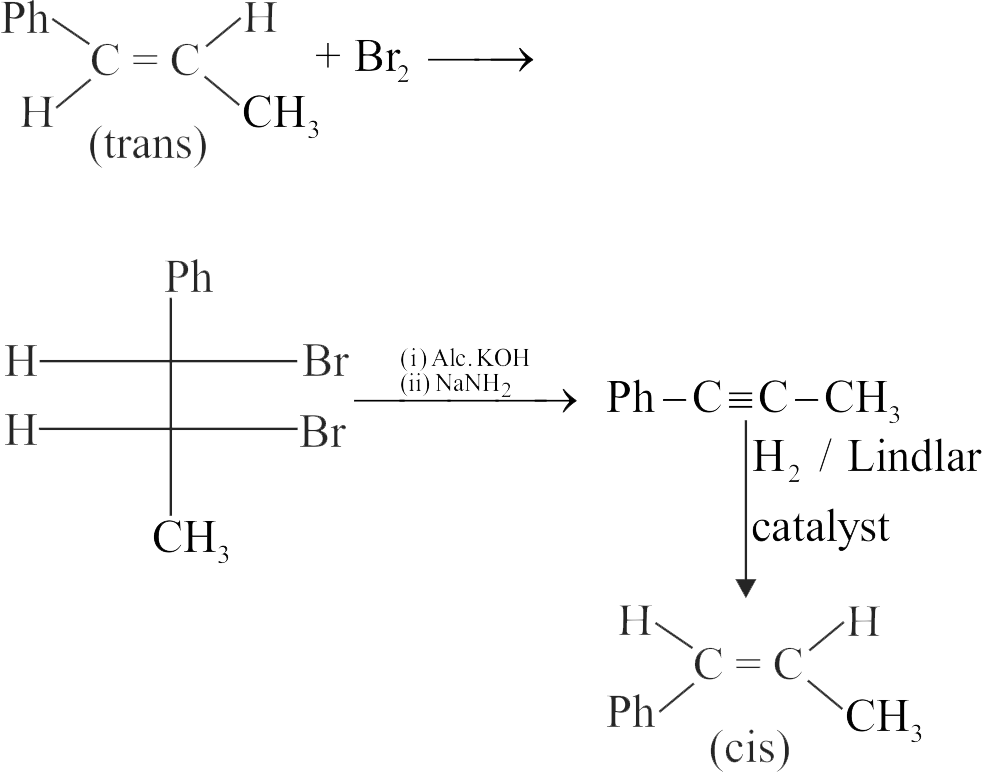
318060
Choose, the correct set of reagents for the following conversion.
\({\mathop{\rm trans}\nolimits} \left( {{\rm{Ph}} - {\rm{CH}} = {\rm{CH}} - {\rm{C}}{{\rm{H}}_3}} \right) \to \)
\({\mathop{\rm cis}\nolimits} \left( {{\rm{Ph}} - {\rm{CH}} = {\rm{CH}} - {\rm{C}}{{\rm{H}}_3}} \right)\)
318060
Choose, the correct set of reagents for the following conversion.
\({\mathop{\rm trans}\nolimits} \left( {{\rm{Ph}} - {\rm{CH}} = {\rm{CH}} - {\rm{C}}{{\rm{H}}_3}} \right) \to \)
\({\mathop{\rm cis}\nolimits} \left( {{\rm{Ph}} - {\rm{CH}} = {\rm{CH}} - {\rm{C}}{{\rm{H}}_3}} \right)\)
318060
Choose, the correct set of reagents for the following conversion.
\({\mathop{\rm trans}\nolimits} \left( {{\rm{Ph}} - {\rm{CH}} = {\rm{CH}} - {\rm{C}}{{\rm{H}}_3}} \right) \to \)
\({\mathop{\rm cis}\nolimits} \left( {{\rm{Ph}} - {\rm{CH}} = {\rm{CH}} - {\rm{C}}{{\rm{H}}_3}} \right)\)
318060
Choose, the correct set of reagents for the following conversion.
\({\mathop{\rm trans}\nolimits} \left( {{\rm{Ph}} - {\rm{CH}} = {\rm{CH}} - {\rm{C}}{{\rm{H}}_3}} \right) \to \)
\({\mathop{\rm cis}\nolimits} \left( {{\rm{Ph}} - {\rm{CH}} = {\rm{CH}} - {\rm{C}}{{\rm{H}}_3}} \right)\)
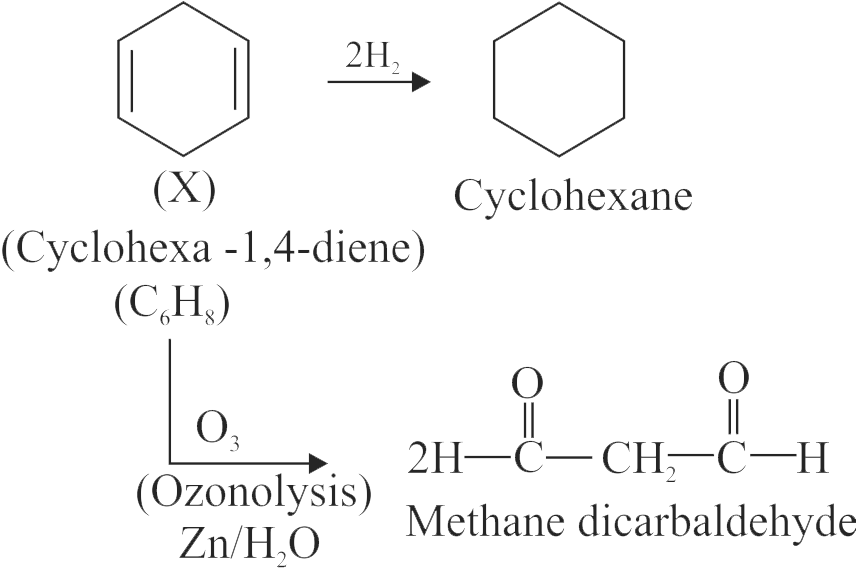
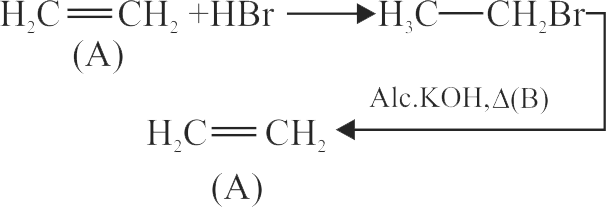
.jpg)
.jpg)
.jpg)