CHXI13:HYDROCARBONS
317996
Assertion :
Ethene on treating with in presence of forms and .
Reason :
This addition involves the formation of free radicals.
1 Both Assertion and Reason are correct and Reason is the correct explanation of the Assertion.
2 Both Assertion and Reason are correct but Reason is not the correct explanation of the Assertion.
3 Assertion is correct but Reason is incorrect.
4 Assertion is incorrect but Reason is correct.
Explanation:
The addition of follows ionic mechanism,
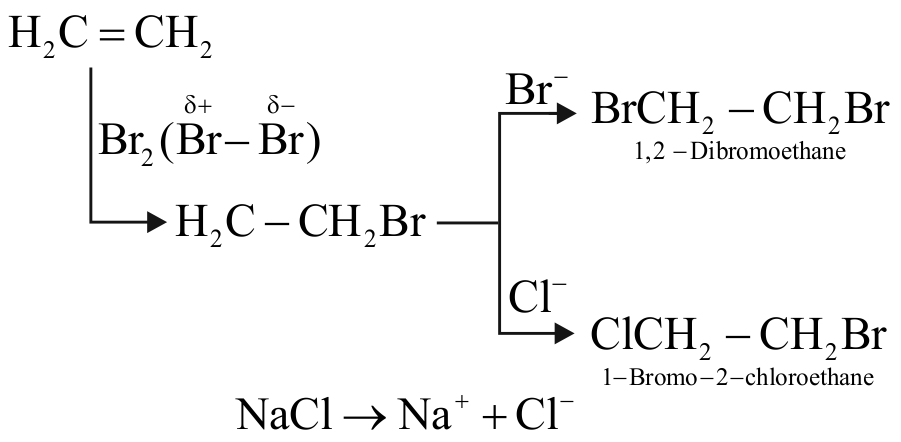
So, option (3) is correct.