317963
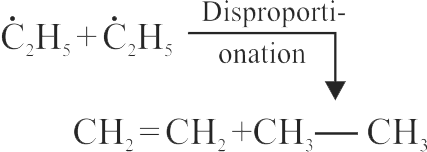
Find out the number of dimerized products obtain by following reaction.
\[\begin{gathered}
{{\text{H}}_{\text{3}}}{\text{C}} - {\text{Cl}} + {{\text{H}}_{\text{3}}}{\text{C}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{Cl}} + \hfill \\
{{\text{H}}_{\text{3}}}{\text{C}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{Cl}}\xrightarrow[{{\text{Dry}}\,\,{\text{ether}}}]{{{\text{Na}}}} \hfill \\
\end{gathered} \]
317963
Find out the number of dimerized products obtain by following reaction.
\[\begin{gathered}
{{\text{H}}_{\text{3}}}{\text{C}} - {\text{Cl}} + {{\text{H}}_{\text{3}}}{\text{C}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{Cl}} + \hfill \\
{{\text{H}}_{\text{3}}}{\text{C}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{Cl}}\xrightarrow[{{\text{Dry}}\,\,{\text{ether}}}]{{{\text{Na}}}} \hfill \\
\end{gathered} \]
317963
Find out the number of dimerized products obtain by following reaction.
\[\begin{gathered}
{{\text{H}}_{\text{3}}}{\text{C}} - {\text{Cl}} + {{\text{H}}_{\text{3}}}{\text{C}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{Cl}} + \hfill \\
{{\text{H}}_{\text{3}}}{\text{C}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{Cl}}\xrightarrow[{{\text{Dry}}\,\,{\text{ether}}}]{{{\text{Na}}}} \hfill \\
\end{gathered} \]
317963
Find out the number of dimerized products obtain by following reaction.
\[\begin{gathered}
{{\text{H}}_{\text{3}}}{\text{C}} - {\text{Cl}} + {{\text{H}}_{\text{3}}}{\text{C}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{Cl}} + \hfill \\
{{\text{H}}_{\text{3}}}{\text{C}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{Cl}}\xrightarrow[{{\text{Dry}}\,\,{\text{ether}}}]{{{\text{Na}}}} \hfill \\
\end{gathered} \]
317963
Find out the number of dimerized products obtain by following reaction.
\[\begin{gathered}
{{\text{H}}_{\text{3}}}{\text{C}} - {\text{Cl}} + {{\text{H}}_{\text{3}}}{\text{C}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{Cl}} + \hfill \\
{{\text{H}}_{\text{3}}}{\text{C}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{Cl}}\xrightarrow[{{\text{Dry}}\,\,{\text{ether}}}]{{{\text{Na}}}} \hfill \\
\end{gathered} \]