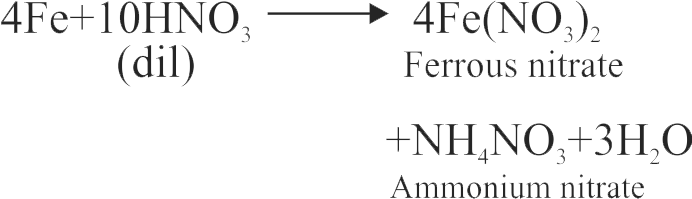316734
Statement A :
When a metal is treated with conc. \(\mathrm{HNO}_{3}\) it generally yields a nitrate, \(\mathrm{NO}_{2}\) and \(\mathrm{H}_{2} \mathrm{O}\).
Statement B :
Conc. \(\mathrm{HNO}_{3}\) reacts with metal and first produces a metal nitrate and nascent hydrogen. The nascent hydrogen then further reduces \(\mathrm{HNO}_{3}\) to \(\mathrm{NO}_{2}\).
316734
Statement A :
When a metal is treated with conc. \(\mathrm{HNO}_{3}\) it generally yields a nitrate, \(\mathrm{NO}_{2}\) and \(\mathrm{H}_{2} \mathrm{O}\).
Statement B :
Conc. \(\mathrm{HNO}_{3}\) reacts with metal and first produces a metal nitrate and nascent hydrogen. The nascent hydrogen then further reduces \(\mathrm{HNO}_{3}\) to \(\mathrm{NO}_{2}\).
316734
Statement A :
When a metal is treated with conc. \(\mathrm{HNO}_{3}\) it generally yields a nitrate, \(\mathrm{NO}_{2}\) and \(\mathrm{H}_{2} \mathrm{O}\).
Statement B :
Conc. \(\mathrm{HNO}_{3}\) reacts with metal and first produces a metal nitrate and nascent hydrogen. The nascent hydrogen then further reduces \(\mathrm{HNO}_{3}\) to \(\mathrm{NO}_{2}\).
316734
Statement A :
When a metal is treated with conc. \(\mathrm{HNO}_{3}\) it generally yields a nitrate, \(\mathrm{NO}_{2}\) and \(\mathrm{H}_{2} \mathrm{O}\).
Statement B :
Conc. \(\mathrm{HNO}_{3}\) reacts with metal and first produces a metal nitrate and nascent hydrogen. The nascent hydrogen then further reduces \(\mathrm{HNO}_{3}\) to \(\mathrm{NO}_{2}\).
316734
Statement A :
When a metal is treated with conc. \(\mathrm{HNO}_{3}\) it generally yields a nitrate, \(\mathrm{NO}_{2}\) and \(\mathrm{H}_{2} \mathrm{O}\).
Statement B :
Conc. \(\mathrm{HNO}_{3}\) reacts with metal and first produces a metal nitrate and nascent hydrogen. The nascent hydrogen then further reduces \(\mathrm{HNO}_{3}\) to \(\mathrm{NO}_{2}\).