316700
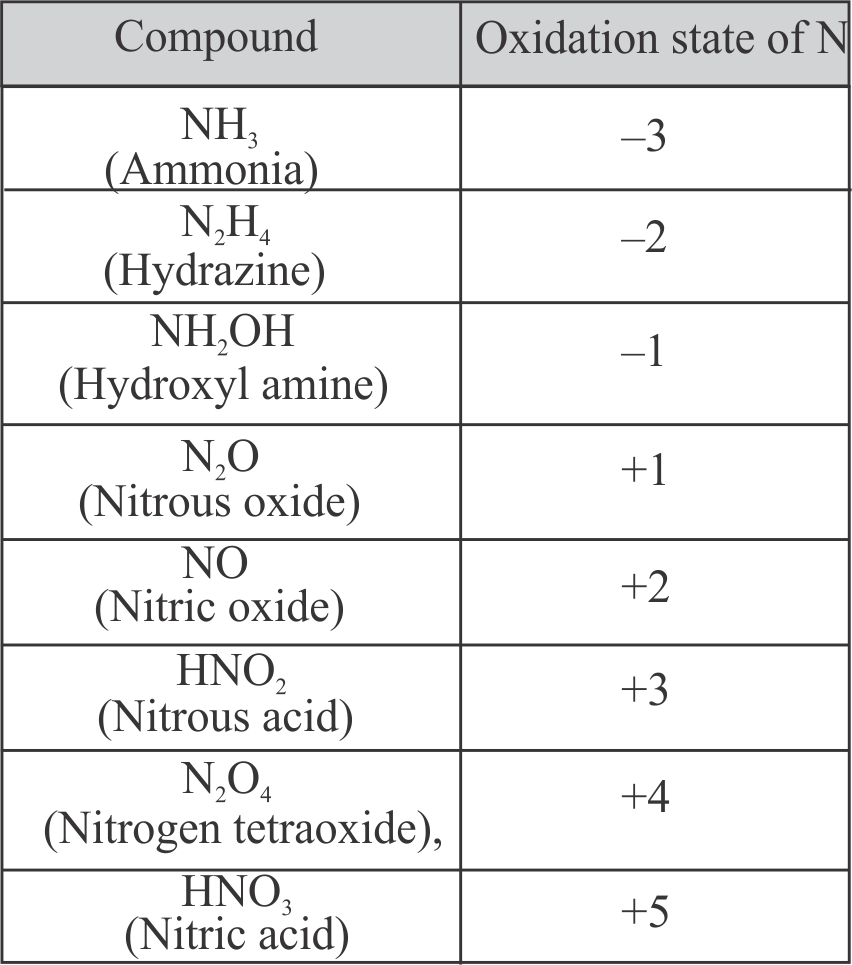
The number of species in the following having positive oxidation states of nitrogen is ____ .
\({\mathrm{\mathrm{NH}_{3}, \mathrm{~N}_{2} \mathrm{O}, \mathrm{NH}_{2} \mathrm{OH}, \mathrm{NO}, \mathrm{HNO}_{2}, \mathrm{~N}_{2} \mathrm{O}_{4}, \mathrm{~N}_{2} \mathrm{H}_{4}}}\), \({\mathrm{\mathrm{HNO}_{3}}}\)
316700
The number of species in the following having positive oxidation states of nitrogen is ____ .
\({\mathrm{\mathrm{NH}_{3}, \mathrm{~N}_{2} \mathrm{O}, \mathrm{NH}_{2} \mathrm{OH}, \mathrm{NO}, \mathrm{HNO}_{2}, \mathrm{~N}_{2} \mathrm{O}_{4}, \mathrm{~N}_{2} \mathrm{H}_{4}}}\), \({\mathrm{\mathrm{HNO}_{3}}}\)
316700
The number of species in the following having positive oxidation states of nitrogen is ____ .
\({\mathrm{\mathrm{NH}_{3}, \mathrm{~N}_{2} \mathrm{O}, \mathrm{NH}_{2} \mathrm{OH}, \mathrm{NO}, \mathrm{HNO}_{2}, \mathrm{~N}_{2} \mathrm{O}_{4}, \mathrm{~N}_{2} \mathrm{H}_{4}}}\), \({\mathrm{\mathrm{HNO}_{3}}}\)
316700
The number of species in the following having positive oxidation states of nitrogen is ____ .
\({\mathrm{\mathrm{NH}_{3}, \mathrm{~N}_{2} \mathrm{O}, \mathrm{NH}_{2} \mathrm{OH}, \mathrm{NO}, \mathrm{HNO}_{2}, \mathrm{~N}_{2} \mathrm{O}_{4}, \mathrm{~N}_{2} \mathrm{H}_{4}}}\), \({\mathrm{\mathrm{HNO}_{3}}}\)
316700
The number of species in the following having positive oxidation states of nitrogen is ____ .
\({\mathrm{\mathrm{NH}_{3}, \mathrm{~N}_{2} \mathrm{O}, \mathrm{NH}_{2} \mathrm{OH}, \mathrm{NO}, \mathrm{HNO}_{2}, \mathrm{~N}_{2} \mathrm{O}_{4}, \mathrm{~N}_{2} \mathrm{H}_{4}}}\), \({\mathrm{\mathrm{HNO}_{3}}}\)