Explanation:
A peroxyl acid is one that contain peroxide \([-\mathrm{O}-\mathrm{O}-\) ] linkage. As perchloric acid \(\left(\mathrm{HClO}_{4}\right)\) doesn't contain a peroxide linkage, it is not a peroxyl acid, whereas others having a peroxide linkage are peroxyl acid.
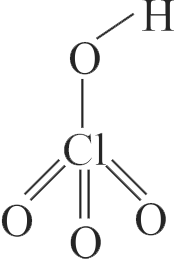
\(\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\rm{Structure}}\,\,{\rm{of }}\,{\rm{HCl}}{{\rm{O}}_{\rm{4}}}\)