316459
Consider the following statements regarding preparation of interhalogen compounds.
I. These can be prepared by the direct combination.
II. These can be prepared by the action of halogen on lower interhalogen compounds.
III. The product formed depends upon some specific conditions.
The correct set of statements is
316443
Match Column I with Column II and choose the correct combination from the options given.
Column I
Column II
A
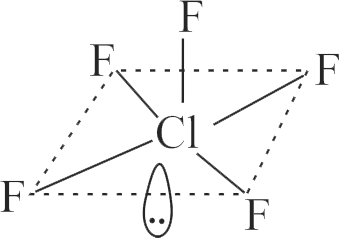
\({\text{CI}}{{\text{F}}_{\text{3}}}\)
P
Trigonal planar
B
\({\rm{I}}{{\rm{F}}_7}\)
Q
Bent T-shape
C
\({\rm{CI}}{{\rm{F}}_5}\)
R
Pentagonal bipyramidal
D
\({\rm{Br}}\)
S
Square pyramidal
316459
Consider the following statements regarding preparation of interhalogen compounds.
I. These can be prepared by the direct combination.
II. These can be prepared by the action of halogen on lower interhalogen compounds.
III. The product formed depends upon some specific conditions.
The correct set of statements is
316443
Match Column I with Column II and choose the correct combination from the options given.
Column I
Column II
A
\({\text{CI}}{{\text{F}}_{\text{3}}}\)
P
Trigonal planar
B
\({\rm{I}}{{\rm{F}}_7}\)
Q
Bent T-shape
C
\({\rm{CI}}{{\rm{F}}_5}\)
R
Pentagonal bipyramidal
D
\({\rm{Br}}\)
S
Square pyramidal
316459
Consider the following statements regarding preparation of interhalogen compounds.
I. These can be prepared by the direct combination.
II. These can be prepared by the action of halogen on lower interhalogen compounds.
III. The product formed depends upon some specific conditions.
The correct set of statements is
316443
Match Column I with Column II and choose the correct combination from the options given.
Column I
Column II
A
\({\text{CI}}{{\text{F}}_{\text{3}}}\)
P
Trigonal planar
B
\({\rm{I}}{{\rm{F}}_7}\)
Q
Bent T-shape
C
\({\rm{CI}}{{\rm{F}}_5}\)
R
Pentagonal bipyramidal
D
\({\rm{Br}}\)
S
Square pyramidal
316459
Consider the following statements regarding preparation of interhalogen compounds.
I. These can be prepared by the direct combination.
II. These can be prepared by the action of halogen on lower interhalogen compounds.
III. The product formed depends upon some specific conditions.
The correct set of statements is
316443
Match Column I with Column II and choose the correct combination from the options given.
Column I
Column II
A
\({\text{CI}}{{\text{F}}_{\text{3}}}\)
P
Trigonal planar
B
\({\rm{I}}{{\rm{F}}_7}\)
Q
Bent T-shape
C
\({\rm{CI}}{{\rm{F}}_5}\)
R
Pentagonal bipyramidal
D
\({\rm{Br}}\)
S
Square pyramidal
316459
Consider the following statements regarding preparation of interhalogen compounds.
I. These can be prepared by the direct combination.
II. These can be prepared by the action of halogen on lower interhalogen compounds.
III. The product formed depends upon some specific conditions.
The correct set of statements is
316443
Match Column I with Column II and choose the correct combination from the options given.
Column I
Column II
A
\({\text{CI}}{{\text{F}}_{\text{3}}}\)
P
Trigonal planar
B
\({\rm{I}}{{\rm{F}}_7}\)
Q
Bent T-shape
C
\({\rm{CI}}{{\rm{F}}_5}\)
R
Pentagonal bipyramidal
D
\({\rm{Br}}\)
S
Square pyramidal