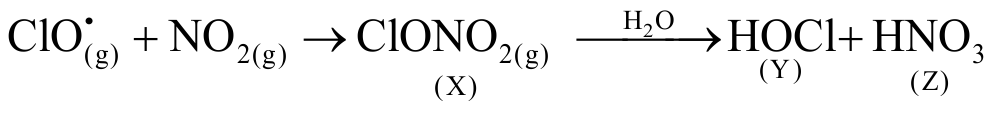316281
Read Statement A and Statement B carefully and mark the correct option.
Statement A :
Chlorine can easily combine with oxygen to form oxides, and the product has a tendency to explode.
Statement B :
Chemical reactivity of an element can be determined by its reaction with oxygen and halogens.
316281
Read Statement A and Statement B carefully and mark the correct option.
Statement A :
Chlorine can easily combine with oxygen to form oxides, and the product has a tendency to explode.
Statement B :
Chemical reactivity of an element can be determined by its reaction with oxygen and halogens.
316281
Read Statement A and Statement B carefully and mark the correct option.
Statement A :
Chlorine can easily combine with oxygen to form oxides, and the product has a tendency to explode.
Statement B :
Chemical reactivity of an element can be determined by its reaction with oxygen and halogens.
316281
Read Statement A and Statement B carefully and mark the correct option.
Statement A :
Chlorine can easily combine with oxygen to form oxides, and the product has a tendency to explode.
Statement B :
Chemical reactivity of an element can be determined by its reaction with oxygen and halogens.
316281
Read Statement A and Statement B carefully and mark the correct option.
Statement A :
Chlorine can easily combine with oxygen to form oxides, and the product has a tendency to explode.
Statement B :
Chemical reactivity of an element can be determined by its reaction with oxygen and halogens.