316143
Match the column I with column II
Choose the correct answer from the options given below
Column I
Column II
A
Coke
P
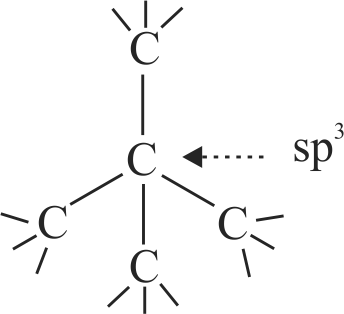
Carbon atoms are\({\text{ s}}{{\text{p}}^{\text{3}}}\) hybridised
B
Diamond
Q
Used as a dry lubricant
C
Fullerene
R
Used as a reducing agent
D
Graphite
S
Cage like molecules
316144
Consider the following statements about graphite.
I. It has layered structure.
II. These layers are held by van der Waal's forces and distance between two layers is 340 pm.
III. Each layer is composed of planar hexagonal rings of carbon atoms.
IV. C-C bond length within the layer is 141.5 \(\mathrm{pm}\).
The correct statements are
316143
Match the column I with column II
Choose the correct answer from the options given below
Column I
Column II
A
Coke
P
Carbon atoms are\({\text{ s}}{{\text{p}}^{\text{3}}}\) hybridised
B
Diamond
Q
Used as a dry lubricant
C
Fullerene
R
Used as a reducing agent
D
Graphite
S
Cage like molecules
316144
Consider the following statements about graphite.
I. It has layered structure.
II. These layers are held by van der Waal's forces and distance between two layers is 340 pm.
III. Each layer is composed of planar hexagonal rings of carbon atoms.
IV. C-C bond length within the layer is 141.5 \(\mathrm{pm}\).
The correct statements are
316143
Match the column I with column II
Choose the correct answer from the options given below
Column I
Column II
A
Coke
P
Carbon atoms are\({\text{ s}}{{\text{p}}^{\text{3}}}\) hybridised
B
Diamond
Q
Used as a dry lubricant
C
Fullerene
R
Used as a reducing agent
D
Graphite
S
Cage like molecules
316144
Consider the following statements about graphite.
I. It has layered structure.
II. These layers are held by van der Waal's forces and distance between two layers is 340 pm.
III. Each layer is composed of planar hexagonal rings of carbon atoms.
IV. C-C bond length within the layer is 141.5 \(\mathrm{pm}\).
The correct statements are
316143
Match the column I with column II
Choose the correct answer from the options given below
Column I
Column II
A
Coke
P
Carbon atoms are\({\text{ s}}{{\text{p}}^{\text{3}}}\) hybridised
B
Diamond
Q
Used as a dry lubricant
C
Fullerene
R
Used as a reducing agent
D
Graphite
S
Cage like molecules
316144
Consider the following statements about graphite.
I. It has layered structure.
II. These layers are held by van der Waal's forces and distance between two layers is 340 pm.
III. Each layer is composed of planar hexagonal rings of carbon atoms.
IV. C-C bond length within the layer is 141.5 \(\mathrm{pm}\).
The correct statements are