316115
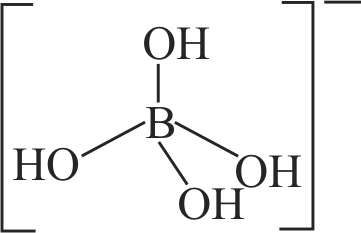
The geometry of a complex species can be understood from the knowledge of type of hybridisation of orbitals of central atom. The hybridisation of orbitals of central atom in
\(\left[\mathrm{B}\left(\mathrm{OH}_{4}\right)\right]^{-}\)and the geometry of the complex are respectively
316115
The geometry of a complex species can be understood from the knowledge of type of hybridisation of orbitals of central atom. The hybridisation of orbitals of central atom in
\(\left[\mathrm{B}\left(\mathrm{OH}_{4}\right)\right]^{-}\)and the geometry of the complex are respectively
316115
The geometry of a complex species can be understood from the knowledge of type of hybridisation of orbitals of central atom. The hybridisation of orbitals of central atom in
\(\left[\mathrm{B}\left(\mathrm{OH}_{4}\right)\right]^{-}\)and the geometry of the complex are respectively
316115
The geometry of a complex species can be understood from the knowledge of type of hybridisation of orbitals of central atom. The hybridisation of orbitals of central atom in
\(\left[\mathrm{B}\left(\mathrm{OH}_{4}\right)\right]^{-}\)and the geometry of the complex are respectively