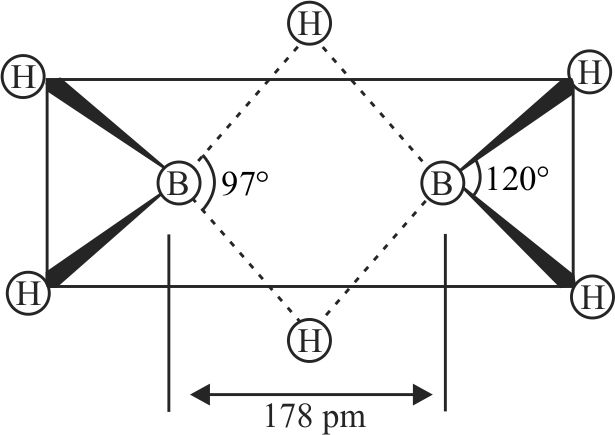
316078 When an inorganic compound \((\mathrm{X})\) having \(3 \mathrm{c}-2 \mathrm{e}\) as well as \(2 \mathrm{c}-2 \mathrm{e}\) bonds reacts with ammonia gas at a certain temperature, gives a compound \((\mathrm{Y})\), isostructural with benzene. Compound (X) with ammonia at a high temperature produces a substance (Z). Identify the correct statement.
316078 When an inorganic compound \((\mathrm{X})\) having \(3 \mathrm{c}-2 \mathrm{e}\) as well as \(2 \mathrm{c}-2 \mathrm{e}\) bonds reacts with ammonia gas at a certain temperature, gives a compound \((\mathrm{Y})\), isostructural with benzene. Compound (X) with ammonia at a high temperature produces a substance (Z). Identify the correct statement.
316078 When an inorganic compound \((\mathrm{X})\) having \(3 \mathrm{c}-2 \mathrm{e}\) as well as \(2 \mathrm{c}-2 \mathrm{e}\) bonds reacts with ammonia gas at a certain temperature, gives a compound \((\mathrm{Y})\), isostructural with benzene. Compound (X) with ammonia at a high temperature produces a substance (Z). Identify the correct statement.
316078 When an inorganic compound \((\mathrm{X})\) having \(3 \mathrm{c}-2 \mathrm{e}\) as well as \(2 \mathrm{c}-2 \mathrm{e}\) bonds reacts with ammonia gas at a certain temperature, gives a compound \((\mathrm{Y})\), isostructural with benzene. Compound (X) with ammonia at a high temperature produces a substance (Z). Identify the correct statement.
316078 When an inorganic compound \((\mathrm{X})\) having \(3 \mathrm{c}-2 \mathrm{e}\) as well as \(2 \mathrm{c}-2 \mathrm{e}\) bonds reacts with ammonia gas at a certain temperature, gives a compound \((\mathrm{Y})\), isostructural with benzene. Compound (X) with ammonia at a high temperature produces a substance (Z). Identify the correct statement.