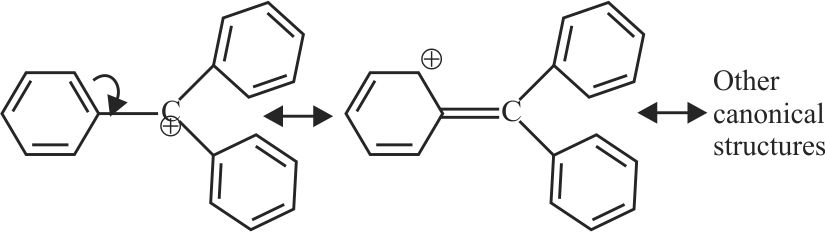
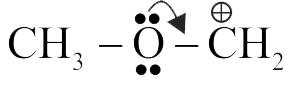317445
Consider the following carbocations :
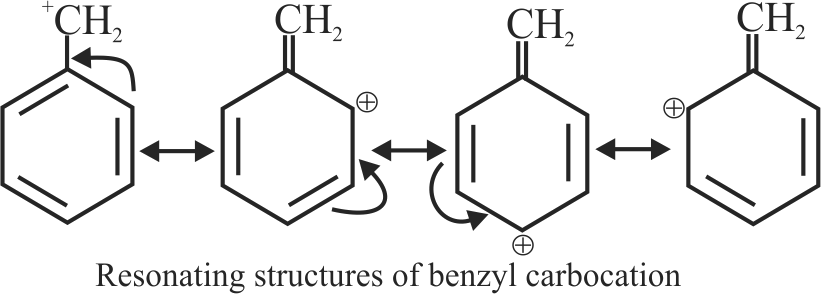
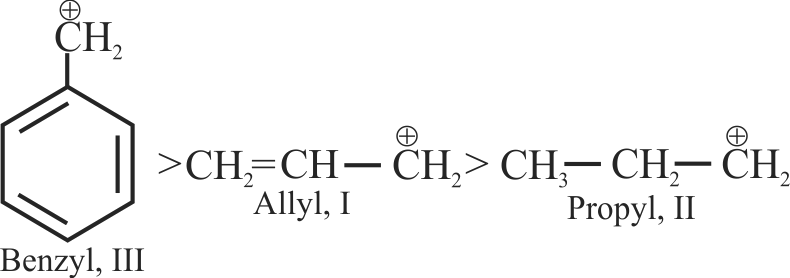
I. \(\mathrm{C}_{6} \mathrm{H}_{5} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\)
II. \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\)
III. \(\mathrm{C}_{6} \mathrm{H}_{5} \stackrel{+}{\mathrm{C}} \mathrm{HCH}_{3}\)
IV. \(\mathrm{C}_{6} \mathrm{H}_{5} \stackrel{+}{\mathrm{C}}\left(\mathrm{CH}_{3}\right)_{2}\)
The correct sequence for the stability of these carbocations is
317447
Consider the following statements:
(I) \(\mathrm{CH}_{3} \mathrm{O} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\) is more stable than \(\mathrm{CH}_{3} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\).
(II) \(\mathrm{Me}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}\) is more stable than \(\mathrm{CH}_{3} \mathrm{CH}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\).
(III) \(\mathrm{CH}_{2}=\mathrm{CH}-\stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\) is more stable than\(\mathrm{CH}_{3} \mathrm{CH}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2} .\)
(IV) \(\mathrm{CH}_{2}=\stackrel{+}{\mathrm{C}} \mathrm{H}\) is more stable than \(\mathrm{CH}_{3} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\). of these statements:
317445
Consider the following carbocations :
I. \(\mathrm{C}_{6} \mathrm{H}_{5} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\)
II. \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\)
III. \(\mathrm{C}_{6} \mathrm{H}_{5} \stackrel{+}{\mathrm{C}} \mathrm{HCH}_{3}\)
IV. \(\mathrm{C}_{6} \mathrm{H}_{5} \stackrel{+}{\mathrm{C}}\left(\mathrm{CH}_{3}\right)_{2}\)
The correct sequence for the stability of these carbocations is
317447
Consider the following statements:
(I) \(\mathrm{CH}_{3} \mathrm{O} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\) is more stable than \(\mathrm{CH}_{3} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\).
(II) \(\mathrm{Me}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}\) is more stable than \(\mathrm{CH}_{3} \mathrm{CH}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\).
(III) \(\mathrm{CH}_{2}=\mathrm{CH}-\stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\) is more stable than\(\mathrm{CH}_{3} \mathrm{CH}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2} .\)
(IV) \(\mathrm{CH}_{2}=\stackrel{+}{\mathrm{C}} \mathrm{H}\) is more stable than \(\mathrm{CH}_{3} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\). of these statements:
317445
Consider the following carbocations :
I. \(\mathrm{C}_{6} \mathrm{H}_{5} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\)
II. \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\)
III. \(\mathrm{C}_{6} \mathrm{H}_{5} \stackrel{+}{\mathrm{C}} \mathrm{HCH}_{3}\)
IV. \(\mathrm{C}_{6} \mathrm{H}_{5} \stackrel{+}{\mathrm{C}}\left(\mathrm{CH}_{3}\right)_{2}\)
The correct sequence for the stability of these carbocations is
317447
Consider the following statements:
(I) \(\mathrm{CH}_{3} \mathrm{O} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\) is more stable than \(\mathrm{CH}_{3} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\).
(II) \(\mathrm{Me}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}\) is more stable than \(\mathrm{CH}_{3} \mathrm{CH}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\).
(III) \(\mathrm{CH}_{2}=\mathrm{CH}-\stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\) is more stable than\(\mathrm{CH}_{3} \mathrm{CH}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2} .\)
(IV) \(\mathrm{CH}_{2}=\stackrel{+}{\mathrm{C}} \mathrm{H}\) is more stable than \(\mathrm{CH}_{3} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\). of these statements:
317445
Consider the following carbocations :
I. \(\mathrm{C}_{6} \mathrm{H}_{5} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\)
II. \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\)
III. \(\mathrm{C}_{6} \mathrm{H}_{5} \stackrel{+}{\mathrm{C}} \mathrm{HCH}_{3}\)
IV. \(\mathrm{C}_{6} \mathrm{H}_{5} \stackrel{+}{\mathrm{C}}\left(\mathrm{CH}_{3}\right)_{2}\)
The correct sequence for the stability of these carbocations is
317447
Consider the following statements:
(I) \(\mathrm{CH}_{3} \mathrm{O} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\) is more stable than \(\mathrm{CH}_{3} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\).
(II) \(\mathrm{Me}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}\) is more stable than \(\mathrm{CH}_{3} \mathrm{CH}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\).
(III) \(\mathrm{CH}_{2}=\mathrm{CH}-\stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\) is more stable than\(\mathrm{CH}_{3} \mathrm{CH}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2} .\)
(IV) \(\mathrm{CH}_{2}=\stackrel{+}{\mathrm{C}} \mathrm{H}\) is more stable than \(\mathrm{CH}_{3} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\). of these statements:
317445
Consider the following carbocations :
I. \(\mathrm{C}_{6} \mathrm{H}_{5} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\)
II. \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\)
III. \(\mathrm{C}_{6} \mathrm{H}_{5} \stackrel{+}{\mathrm{C}} \mathrm{HCH}_{3}\)
IV. \(\mathrm{C}_{6} \mathrm{H}_{5} \stackrel{+}{\mathrm{C}}\left(\mathrm{CH}_{3}\right)_{2}\)
The correct sequence for the stability of these carbocations is
317447
Consider the following statements:
(I) \(\mathrm{CH}_{3} \mathrm{O} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\) is more stable than \(\mathrm{CH}_{3} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\).
(II) \(\mathrm{Me}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}\) is more stable than \(\mathrm{CH}_{3} \mathrm{CH}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\).
(III) \(\mathrm{CH}_{2}=\mathrm{CH}-\stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\) is more stable than\(\mathrm{CH}_{3} \mathrm{CH}_{2} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2} .\)
(IV) \(\mathrm{CH}_{2}=\stackrel{+}{\mathrm{C}} \mathrm{H}\) is more stable than \(\mathrm{CH}_{3} \stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\). of these statements:
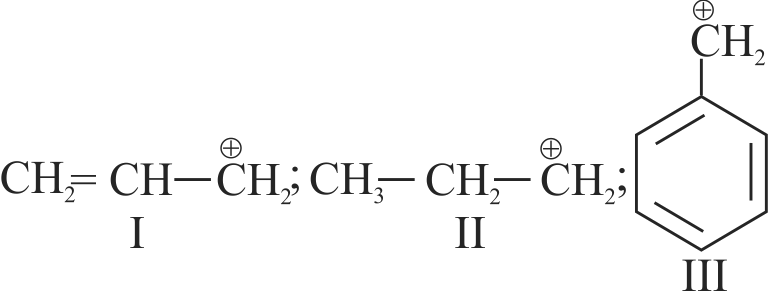
.png)