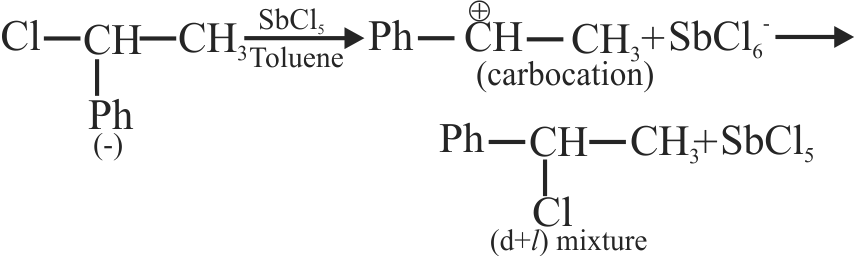
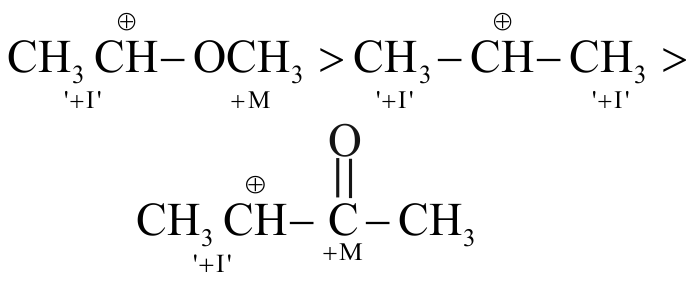317442
What is the correct order of decreasing stability of the following cations.
(I) \(\mathrm{CH}_{3}-\stackrel{\oplus}{\mathrm{C}} \mathrm{H}-\mathrm{CH}_{3}\)
(II) \(\mathrm{CH}_{3}-\stackrel{\oplus}{\mathrm{C}} \mathrm{H}-\mathrm{OCH}_{3}\)
(III) \(\mathrm{CH}_{3}-\stackrel{\oplus}{\mathrm{C}} \mathrm{H}-\mathrm{CH}_{2}-\mathrm{OCH}_{3}\)
317443
The decreasing order of the stability of ions,
\({\text{C}}{{\text{H}}_3} - \mathop {\mathop {\text{C}}\limits^ + }\limits_{\text{I}} {\text{H}} - {\text{C}}{{\text{H}}_3}{\text{ C}}{{\text{H}}_3} - \mathop {\mathop {\text{CH}}\limits^ + }\limits_{{\text{II}}} - {\text{OC}}{{\text{H}}_3}\)
\({\text{C}}{{\text{H}}_3} - \mathop {\mathop {\text{CH}}\limits^ + - {\text{COC}}{{\text{H}}_3}}\limits_{{\text{ III }}} \)
317442
What is the correct order of decreasing stability of the following cations.
(I) \(\mathrm{CH}_{3}-\stackrel{\oplus}{\mathrm{C}} \mathrm{H}-\mathrm{CH}_{3}\)
(II) \(\mathrm{CH}_{3}-\stackrel{\oplus}{\mathrm{C}} \mathrm{H}-\mathrm{OCH}_{3}\)
(III) \(\mathrm{CH}_{3}-\stackrel{\oplus}{\mathrm{C}} \mathrm{H}-\mathrm{CH}_{2}-\mathrm{OCH}_{3}\)
317443
The decreasing order of the stability of ions,
\({\text{C}}{{\text{H}}_3} - \mathop {\mathop {\text{C}}\limits^ + }\limits_{\text{I}} {\text{H}} - {\text{C}}{{\text{H}}_3}{\text{ C}}{{\text{H}}_3} - \mathop {\mathop {\text{CH}}\limits^ + }\limits_{{\text{II}}} - {\text{OC}}{{\text{H}}_3}\)
\({\text{C}}{{\text{H}}_3} - \mathop {\mathop {\text{CH}}\limits^ + - {\text{COC}}{{\text{H}}_3}}\limits_{{\text{ III }}} \)
317442
What is the correct order of decreasing stability of the following cations.
(I) \(\mathrm{CH}_{3}-\stackrel{\oplus}{\mathrm{C}} \mathrm{H}-\mathrm{CH}_{3}\)
(II) \(\mathrm{CH}_{3}-\stackrel{\oplus}{\mathrm{C}} \mathrm{H}-\mathrm{OCH}_{3}\)
(III) \(\mathrm{CH}_{3}-\stackrel{\oplus}{\mathrm{C}} \mathrm{H}-\mathrm{CH}_{2}-\mathrm{OCH}_{3}\)
317443
The decreasing order of the stability of ions,
\({\text{C}}{{\text{H}}_3} - \mathop {\mathop {\text{C}}\limits^ + }\limits_{\text{I}} {\text{H}} - {\text{C}}{{\text{H}}_3}{\text{ C}}{{\text{H}}_3} - \mathop {\mathop {\text{CH}}\limits^ + }\limits_{{\text{II}}} - {\text{OC}}{{\text{H}}_3}\)
\({\text{C}}{{\text{H}}_3} - \mathop {\mathop {\text{CH}}\limits^ + - {\text{COC}}{{\text{H}}_3}}\limits_{{\text{ III }}} \)
317442
What is the correct order of decreasing stability of the following cations.
(I) \(\mathrm{CH}_{3}-\stackrel{\oplus}{\mathrm{C}} \mathrm{H}-\mathrm{CH}_{3}\)
(II) \(\mathrm{CH}_{3}-\stackrel{\oplus}{\mathrm{C}} \mathrm{H}-\mathrm{OCH}_{3}\)
(III) \(\mathrm{CH}_{3}-\stackrel{\oplus}{\mathrm{C}} \mathrm{H}-\mathrm{CH}_{2}-\mathrm{OCH}_{3}\)
317443
The decreasing order of the stability of ions,
\({\text{C}}{{\text{H}}_3} - \mathop {\mathop {\text{C}}\limits^ + }\limits_{\text{I}} {\text{H}} - {\text{C}}{{\text{H}}_3}{\text{ C}}{{\text{H}}_3} - \mathop {\mathop {\text{CH}}\limits^ + }\limits_{{\text{II}}} - {\text{OC}}{{\text{H}}_3}\)
\({\text{C}}{{\text{H}}_3} - \mathop {\mathop {\text{CH}}\limits^ + - {\text{COC}}{{\text{H}}_3}}\limits_{{\text{ III }}} \)