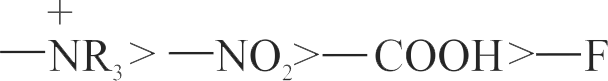
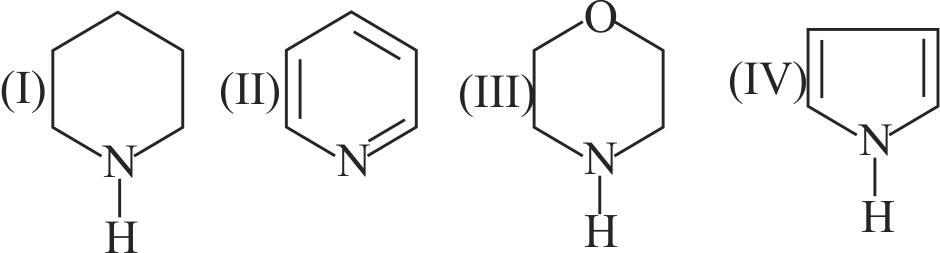
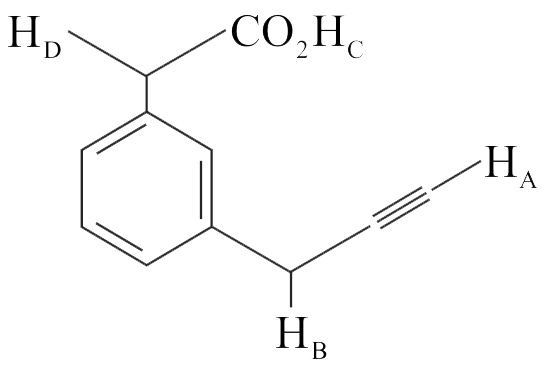
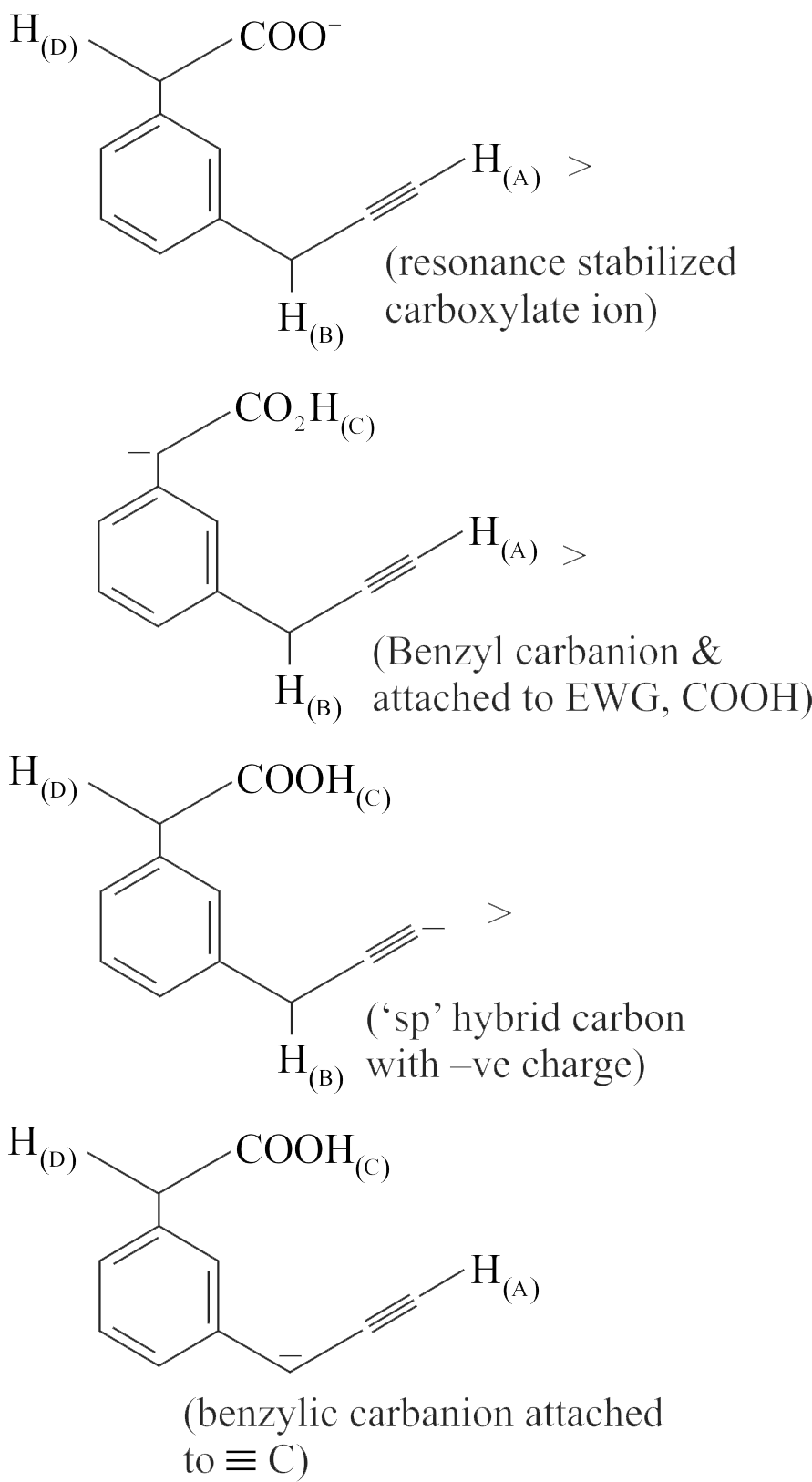
317223
Assertion :
A compound with delocalised electrons is more stable than that compound would be if all its electrons were localised.
Reason :
The extra stability, a compound gains as a result of having delocalised electrons, is called delocalisation energy.
317223
Assertion :
A compound with delocalised electrons is more stable than that compound would be if all its electrons were localised.
Reason :
The extra stability, a compound gains as a result of having delocalised electrons, is called delocalisation energy.
317223
Assertion :
A compound with delocalised electrons is more stable than that compound would be if all its electrons were localised.
Reason :
The extra stability, a compound gains as a result of having delocalised electrons, is called delocalisation energy.
317223
Assertion :
A compound with delocalised electrons is more stable than that compound would be if all its electrons were localised.
Reason :
The extra stability, a compound gains as a result of having delocalised electrons, is called delocalisation energy.