317196
Arrange the following compounds in the order of decreasing \(\mathrm{K}_{\mathrm{a}}\).
(P) \(\mathrm{F}-\mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOH}\)
(Q)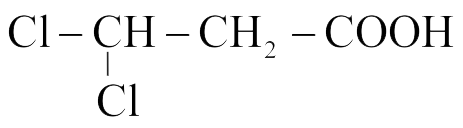
(R) \(\mathrm{F}-\mathrm{CH}_{2}-\mathrm{COOH}\)
(S) \(\mathrm{Br}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{COOH}\)
Correct answer is
317196
Arrange the following compounds in the order of decreasing \(\mathrm{K}_{\mathrm{a}}\).
(P) \(\mathrm{F}-\mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOH}\)
(Q)
(R) \(\mathrm{F}-\mathrm{CH}_{2}-\mathrm{COOH}\)
(S) \(\mathrm{Br}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{COOH}\)
Correct answer is
317196
Arrange the following compounds in the order of decreasing \(\mathrm{K}_{\mathrm{a}}\).
(P) \(\mathrm{F}-\mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOH}\)
(Q)
(R) \(\mathrm{F}-\mathrm{CH}_{2}-\mathrm{COOH}\)
(S) \(\mathrm{Br}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{COOH}\)
Correct answer is
317196
Arrange the following compounds in the order of decreasing \(\mathrm{K}_{\mathrm{a}}\).
(P) \(\mathrm{F}-\mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOH}\)
(Q)
(R) \(\mathrm{F}-\mathrm{CH}_{2}-\mathrm{COOH}\)
(S) \(\mathrm{Br}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{COOH}\)
Correct answer is
317196
Arrange the following compounds in the order of decreasing \(\mathrm{K}_{\mathrm{a}}\).
(P) \(\mathrm{F}-\mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOH}\)
(Q)
(R) \(\mathrm{F}-\mathrm{CH}_{2}-\mathrm{COOH}\)
(S) \(\mathrm{Br}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{COOH}\)
Correct answer is