315458
Statement A :
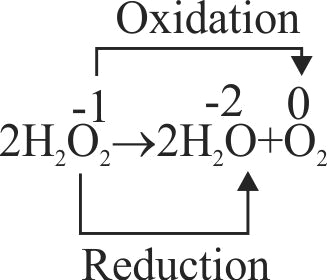
The decomposition of hydrogen peroxide to form water and oxygen is an example of disproportionation reaciton.
Statement B :
The oxygen of peroxide is in \({\rm{ - 1}}\) oxidation state and it is converted to zero oxidation state in \(\mathrm{\mathrm{O}_{2}}\) and \({\rm{ - 2}}\) oxidation state in \(\mathrm{\mathrm{H}_{2} \mathrm{O}}\).
315459
Which of the following reactions are disproportionation reactions?
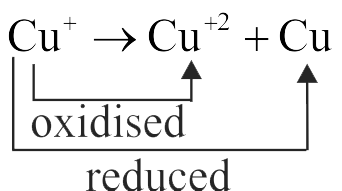
(I) \({\mathrm{\mathrm{Cu}^{+} \rightarrow \mathrm{Cu}^{2+}+\mathrm{Cu}}}\)
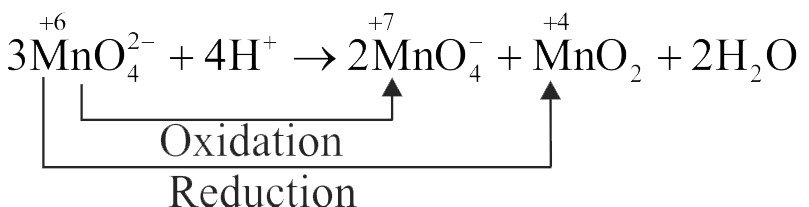
(II) \({\mathrm{3 \mathrm{MnO}_{4}^{2-}+4 \mathrm{H}^{+} \rightarrow 2 \mathrm{MnO}_{4}^{-}+\mathrm{MnO}_{2}+2 \mathrm{H}_{2} \mathrm{O}}}\)
(III) \({\mathrm{2 \mathrm{KMnO}_{4} \rightarrow \mathrm{K}_{2} \mathrm{MnO}_{4}+\mathrm{MnO}_{2}+\mathrm{O}_{2}}}\)
(IV) \({\mathrm{2 \mathrm{MnO}_{4}^{-}+3 \mathrm{Mn}^{2+}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow 5 \mathrm{MnO}_{2}+4 \mathrm{H}^{+}}}\)
Choose the correct answer from the options given below
315460
Chlorine undergoes disproportionation in alkaline medium as shown below
\(\mathrm{aCl}_{2(\mathrm{~g})}+\mathrm{bOH}_{(\mathrm{aq})}^{-} \rightarrow \mathrm{cClO}_{(\mathrm{aq})}^{-}+\mathrm{dCl}_{(\mathrm{aq})}^{-}+\mathrm{eH}_{2} \mathrm{O}_{(\mathrm{l})}\)
The values of \({\mathrm{a, b, c}}\) and \({\mathrm{d}}\) in a balanced redox reaction are respectively
315461
Which of the following reactions are disproportionation reaction?
(a) \(\mathrm{2 \mathrm{Cu}^{+} \longrightarrow \mathrm{Cu}^{2+}+\mathrm{Cu}^{\circ}}\)
(b) \(\mathrm{3 \mathrm{MnO}_{4}^{2-}+4 \mathrm{H}^{+} \longrightarrow 2 \mathrm{MnO}_{4}^{-}+\mathrm{MnO}_{2}+2 \mathrm{H}_{2} \mathrm{O}}\)
(c) \(\mathrm{2 \mathrm{KMnO}_{4} \stackrel{\Delta}{\longrightarrow} \mathrm{K}_{2} \mathrm{MnO}_{4}+\mathrm{MnO}_{2}+\mathrm{O}_{2}}\)
(d) \(\mathrm{2 \mathrm{MnO}_{4}^{-}+3 \mathrm{Mn}^{2+}+2 \mathrm{H}_{2} \mathrm{O} \longrightarrow 5 \mathrm{MnO}_{2}+4 \mathrm{H}^{\oplus}}\)
Select the correct option from the following
315458
Statement A :
The decomposition of hydrogen peroxide to form water and oxygen is an example of disproportionation reaciton.
Statement B :
The oxygen of peroxide is in \({\rm{ - 1}}\) oxidation state and it is converted to zero oxidation state in \(\mathrm{\mathrm{O}_{2}}\) and \({\rm{ - 2}}\) oxidation state in \(\mathrm{\mathrm{H}_{2} \mathrm{O}}\).
315459
Which of the following reactions are disproportionation reactions?
(I) \({\mathrm{\mathrm{Cu}^{+} \rightarrow \mathrm{Cu}^{2+}+\mathrm{Cu}}}\)
(II) \({\mathrm{3 \mathrm{MnO}_{4}^{2-}+4 \mathrm{H}^{+} \rightarrow 2 \mathrm{MnO}_{4}^{-}+\mathrm{MnO}_{2}+2 \mathrm{H}_{2} \mathrm{O}}}\)
(III) \({\mathrm{2 \mathrm{KMnO}_{4} \rightarrow \mathrm{K}_{2} \mathrm{MnO}_{4}+\mathrm{MnO}_{2}+\mathrm{O}_{2}}}\)
(IV) \({\mathrm{2 \mathrm{MnO}_{4}^{-}+3 \mathrm{Mn}^{2+}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow 5 \mathrm{MnO}_{2}+4 \mathrm{H}^{+}}}\)
Choose the correct answer from the options given below
315460
Chlorine undergoes disproportionation in alkaline medium as shown below
\(\mathrm{aCl}_{2(\mathrm{~g})}+\mathrm{bOH}_{(\mathrm{aq})}^{-} \rightarrow \mathrm{cClO}_{(\mathrm{aq})}^{-}+\mathrm{dCl}_{(\mathrm{aq})}^{-}+\mathrm{eH}_{2} \mathrm{O}_{(\mathrm{l})}\)
The values of \({\mathrm{a, b, c}}\) and \({\mathrm{d}}\) in a balanced redox reaction are respectively
315461
Which of the following reactions are disproportionation reaction?
(a) \(\mathrm{2 \mathrm{Cu}^{+} \longrightarrow \mathrm{Cu}^{2+}+\mathrm{Cu}^{\circ}}\)
(b) \(\mathrm{3 \mathrm{MnO}_{4}^{2-}+4 \mathrm{H}^{+} \longrightarrow 2 \mathrm{MnO}_{4}^{-}+\mathrm{MnO}_{2}+2 \mathrm{H}_{2} \mathrm{O}}\)
(c) \(\mathrm{2 \mathrm{KMnO}_{4} \stackrel{\Delta}{\longrightarrow} \mathrm{K}_{2} \mathrm{MnO}_{4}+\mathrm{MnO}_{2}+\mathrm{O}_{2}}\)
(d) \(\mathrm{2 \mathrm{MnO}_{4}^{-}+3 \mathrm{Mn}^{2+}+2 \mathrm{H}_{2} \mathrm{O} \longrightarrow 5 \mathrm{MnO}_{2}+4 \mathrm{H}^{\oplus}}\)
Select the correct option from the following
315458
Statement A :
The decomposition of hydrogen peroxide to form water and oxygen is an example of disproportionation reaciton.
Statement B :
The oxygen of peroxide is in \({\rm{ - 1}}\) oxidation state and it is converted to zero oxidation state in \(\mathrm{\mathrm{O}_{2}}\) and \({\rm{ - 2}}\) oxidation state in \(\mathrm{\mathrm{H}_{2} \mathrm{O}}\).
315459
Which of the following reactions are disproportionation reactions?
(I) \({\mathrm{\mathrm{Cu}^{+} \rightarrow \mathrm{Cu}^{2+}+\mathrm{Cu}}}\)
(II) \({\mathrm{3 \mathrm{MnO}_{4}^{2-}+4 \mathrm{H}^{+} \rightarrow 2 \mathrm{MnO}_{4}^{-}+\mathrm{MnO}_{2}+2 \mathrm{H}_{2} \mathrm{O}}}\)
(III) \({\mathrm{2 \mathrm{KMnO}_{4} \rightarrow \mathrm{K}_{2} \mathrm{MnO}_{4}+\mathrm{MnO}_{2}+\mathrm{O}_{2}}}\)
(IV) \({\mathrm{2 \mathrm{MnO}_{4}^{-}+3 \mathrm{Mn}^{2+}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow 5 \mathrm{MnO}_{2}+4 \mathrm{H}^{+}}}\)
Choose the correct answer from the options given below
315460
Chlorine undergoes disproportionation in alkaline medium as shown below
\(\mathrm{aCl}_{2(\mathrm{~g})}+\mathrm{bOH}_{(\mathrm{aq})}^{-} \rightarrow \mathrm{cClO}_{(\mathrm{aq})}^{-}+\mathrm{dCl}_{(\mathrm{aq})}^{-}+\mathrm{eH}_{2} \mathrm{O}_{(\mathrm{l})}\)
The values of \({\mathrm{a, b, c}}\) and \({\mathrm{d}}\) in a balanced redox reaction are respectively
315461
Which of the following reactions are disproportionation reaction?
(a) \(\mathrm{2 \mathrm{Cu}^{+} \longrightarrow \mathrm{Cu}^{2+}+\mathrm{Cu}^{\circ}}\)
(b) \(\mathrm{3 \mathrm{MnO}_{4}^{2-}+4 \mathrm{H}^{+} \longrightarrow 2 \mathrm{MnO}_{4}^{-}+\mathrm{MnO}_{2}+2 \mathrm{H}_{2} \mathrm{O}}\)
(c) \(\mathrm{2 \mathrm{KMnO}_{4} \stackrel{\Delta}{\longrightarrow} \mathrm{K}_{2} \mathrm{MnO}_{4}+\mathrm{MnO}_{2}+\mathrm{O}_{2}}\)
(d) \(\mathrm{2 \mathrm{MnO}_{4}^{-}+3 \mathrm{Mn}^{2+}+2 \mathrm{H}_{2} \mathrm{O} \longrightarrow 5 \mathrm{MnO}_{2}+4 \mathrm{H}^{\oplus}}\)
Select the correct option from the following
315458
Statement A :
The decomposition of hydrogen peroxide to form water and oxygen is an example of disproportionation reaciton.
Statement B :
The oxygen of peroxide is in \({\rm{ - 1}}\) oxidation state and it is converted to zero oxidation state in \(\mathrm{\mathrm{O}_{2}}\) and \({\rm{ - 2}}\) oxidation state in \(\mathrm{\mathrm{H}_{2} \mathrm{O}}\).
315459
Which of the following reactions are disproportionation reactions?
(I) \({\mathrm{\mathrm{Cu}^{+} \rightarrow \mathrm{Cu}^{2+}+\mathrm{Cu}}}\)
(II) \({\mathrm{3 \mathrm{MnO}_{4}^{2-}+4 \mathrm{H}^{+} \rightarrow 2 \mathrm{MnO}_{4}^{-}+\mathrm{MnO}_{2}+2 \mathrm{H}_{2} \mathrm{O}}}\)
(III) \({\mathrm{2 \mathrm{KMnO}_{4} \rightarrow \mathrm{K}_{2} \mathrm{MnO}_{4}+\mathrm{MnO}_{2}+\mathrm{O}_{2}}}\)
(IV) \({\mathrm{2 \mathrm{MnO}_{4}^{-}+3 \mathrm{Mn}^{2+}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow 5 \mathrm{MnO}_{2}+4 \mathrm{H}^{+}}}\)
Choose the correct answer from the options given below
315460
Chlorine undergoes disproportionation in alkaline medium as shown below
\(\mathrm{aCl}_{2(\mathrm{~g})}+\mathrm{bOH}_{(\mathrm{aq})}^{-} \rightarrow \mathrm{cClO}_{(\mathrm{aq})}^{-}+\mathrm{dCl}_{(\mathrm{aq})}^{-}+\mathrm{eH}_{2} \mathrm{O}_{(\mathrm{l})}\)
The values of \({\mathrm{a, b, c}}\) and \({\mathrm{d}}\) in a balanced redox reaction are respectively
315461
Which of the following reactions are disproportionation reaction?
(a) \(\mathrm{2 \mathrm{Cu}^{+} \longrightarrow \mathrm{Cu}^{2+}+\mathrm{Cu}^{\circ}}\)
(b) \(\mathrm{3 \mathrm{MnO}_{4}^{2-}+4 \mathrm{H}^{+} \longrightarrow 2 \mathrm{MnO}_{4}^{-}+\mathrm{MnO}_{2}+2 \mathrm{H}_{2} \mathrm{O}}\)
(c) \(\mathrm{2 \mathrm{KMnO}_{4} \stackrel{\Delta}{\longrightarrow} \mathrm{K}_{2} \mathrm{MnO}_{4}+\mathrm{MnO}_{2}+\mathrm{O}_{2}}\)
(d) \(\mathrm{2 \mathrm{MnO}_{4}^{-}+3 \mathrm{Mn}^{2+}+2 \mathrm{H}_{2} \mathrm{O} \longrightarrow 5 \mathrm{MnO}_{2}+4 \mathrm{H}^{\oplus}}\)
Select the correct option from the following