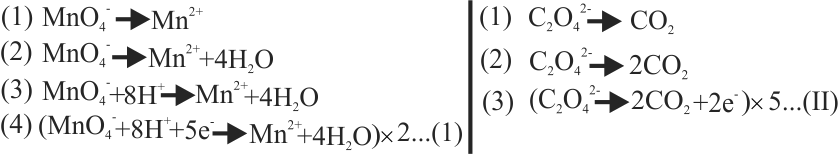315106
For the redox reaction
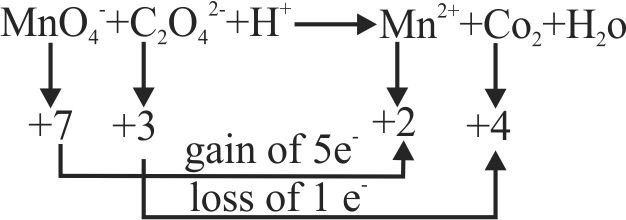
\(\rm{\mathrm{MnO}_{4}^{-}+\mathrm{C}_{2} \mathrm{O}_{4}^{2-}+\mathrm{H}^{+} \rightarrow \mathrm{Mn}^{2+}+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O}}\)
The correct coefficient of the reactants for the balanced equation are
\(\,\,\,\,{\rm{MnO}}_{\rm{4}}^{\rm{ - }}\quad {{\rm{C}}_{\rm{2}}}{\rm{O}}_{\rm{4}}^{{\rm{2 - }}}\quad {{\rm{H}}^{\rm{ + }}}\)
315073
\(\begin{array}{l}
{\rm{F}}{{\rm{e}}^{2 + }} \to {\rm{F}}{{\rm{e}}^{3 + }} + {{\rm{e}}^ - }\\
{\rm{MnO}}_4^ - + {\rm{5}}{{\rm{e}}^ - } \to {\rm{M}}{{\rm{n}}^{2 + }}
\end{array}\)
The ratio of stoichiometric coefficient of \({\rm{F}}{{\rm{e}}^{2 + }}\) and \({\rm{MnO}}_4^ - \)
315076
Dichlorine heptaoxide \(\mathrm{\left(\mathrm{Cl}_{2} \mathrm{O}_{7}\right)}\) in gaseous state combines with an aqueous solution of hydrogen peroxide in acidic medium to give chlorite ion \(\mathrm{\left(\mathrm{ClO}_{2}^{-}\right)}\)and oxygen gas.
The balanced chemical equation for the reaction is
315070
In the redox reaction,
\[\begin{array}{l}
{\rm{xKMn}}{{\rm{O}}_{\rm{4}}} + {\rm{N}}{{\rm{H}}_3} \to {\rm{yKN}}{{\rm{O}}_{\rm{3}}} + {\rm{Mn}}{{\rm{O}}_{\rm{2}}}\\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, + {\rm{KOH}} + {{\rm{H}}_{\rm{2}}}{\rm{O}}
\end{array}\]
x and y are
315106
For the redox reaction
\(\rm{\mathrm{MnO}_{4}^{-}+\mathrm{C}_{2} \mathrm{O}_{4}^{2-}+\mathrm{H}^{+} \rightarrow \mathrm{Mn}^{2+}+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O}}\)
The correct coefficient of the reactants for the balanced equation are
\(\,\,\,\,{\rm{MnO}}_{\rm{4}}^{\rm{ - }}\quad {{\rm{C}}_{\rm{2}}}{\rm{O}}_{\rm{4}}^{{\rm{2 - }}}\quad {{\rm{H}}^{\rm{ + }}}\)
315073
\(\begin{array}{l}
{\rm{F}}{{\rm{e}}^{2 + }} \to {\rm{F}}{{\rm{e}}^{3 + }} + {{\rm{e}}^ - }\\
{\rm{MnO}}_4^ - + {\rm{5}}{{\rm{e}}^ - } \to {\rm{M}}{{\rm{n}}^{2 + }}
\end{array}\)
The ratio of stoichiometric coefficient of \({\rm{F}}{{\rm{e}}^{2 + }}\) and \({\rm{MnO}}_4^ - \)
315076
Dichlorine heptaoxide \(\mathrm{\left(\mathrm{Cl}_{2} \mathrm{O}_{7}\right)}\) in gaseous state combines with an aqueous solution of hydrogen peroxide in acidic medium to give chlorite ion \(\mathrm{\left(\mathrm{ClO}_{2}^{-}\right)}\)and oxygen gas.
The balanced chemical equation for the reaction is
315070
In the redox reaction,
\[\begin{array}{l}
{\rm{xKMn}}{{\rm{O}}_{\rm{4}}} + {\rm{N}}{{\rm{H}}_3} \to {\rm{yKN}}{{\rm{O}}_{\rm{3}}} + {\rm{Mn}}{{\rm{O}}_{\rm{2}}}\\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, + {\rm{KOH}} + {{\rm{H}}_{\rm{2}}}{\rm{O}}
\end{array}\]
x and y are
315106
For the redox reaction
\(\rm{\mathrm{MnO}_{4}^{-}+\mathrm{C}_{2} \mathrm{O}_{4}^{2-}+\mathrm{H}^{+} \rightarrow \mathrm{Mn}^{2+}+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O}}\)
The correct coefficient of the reactants for the balanced equation are
\(\,\,\,\,{\rm{MnO}}_{\rm{4}}^{\rm{ - }}\quad {{\rm{C}}_{\rm{2}}}{\rm{O}}_{\rm{4}}^{{\rm{2 - }}}\quad {{\rm{H}}^{\rm{ + }}}\)
315073
\(\begin{array}{l}
{\rm{F}}{{\rm{e}}^{2 + }} \to {\rm{F}}{{\rm{e}}^{3 + }} + {{\rm{e}}^ - }\\
{\rm{MnO}}_4^ - + {\rm{5}}{{\rm{e}}^ - } \to {\rm{M}}{{\rm{n}}^{2 + }}
\end{array}\)
The ratio of stoichiometric coefficient of \({\rm{F}}{{\rm{e}}^{2 + }}\) and \({\rm{MnO}}_4^ - \)
315076
Dichlorine heptaoxide \(\mathrm{\left(\mathrm{Cl}_{2} \mathrm{O}_{7}\right)}\) in gaseous state combines with an aqueous solution of hydrogen peroxide in acidic medium to give chlorite ion \(\mathrm{\left(\mathrm{ClO}_{2}^{-}\right)}\)and oxygen gas.
The balanced chemical equation for the reaction is
315070
In the redox reaction,
\[\begin{array}{l}
{\rm{xKMn}}{{\rm{O}}_{\rm{4}}} + {\rm{N}}{{\rm{H}}_3} \to {\rm{yKN}}{{\rm{O}}_{\rm{3}}} + {\rm{Mn}}{{\rm{O}}_{\rm{2}}}\\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, + {\rm{KOH}} + {{\rm{H}}_{\rm{2}}}{\rm{O}}
\end{array}\]
x and y are
315106
For the redox reaction
\(\rm{\mathrm{MnO}_{4}^{-}+\mathrm{C}_{2} \mathrm{O}_{4}^{2-}+\mathrm{H}^{+} \rightarrow \mathrm{Mn}^{2+}+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O}}\)
The correct coefficient of the reactants for the balanced equation are
\(\,\,\,\,{\rm{MnO}}_{\rm{4}}^{\rm{ - }}\quad {{\rm{C}}_{\rm{2}}}{\rm{O}}_{\rm{4}}^{{\rm{2 - }}}\quad {{\rm{H}}^{\rm{ + }}}\)
315073
\(\begin{array}{l}
{\rm{F}}{{\rm{e}}^{2 + }} \to {\rm{F}}{{\rm{e}}^{3 + }} + {{\rm{e}}^ - }\\
{\rm{MnO}}_4^ - + {\rm{5}}{{\rm{e}}^ - } \to {\rm{M}}{{\rm{n}}^{2 + }}
\end{array}\)
The ratio of stoichiometric coefficient of \({\rm{F}}{{\rm{e}}^{2 + }}\) and \({\rm{MnO}}_4^ - \)
315076
Dichlorine heptaoxide \(\mathrm{\left(\mathrm{Cl}_{2} \mathrm{O}_{7}\right)}\) in gaseous state combines with an aqueous solution of hydrogen peroxide in acidic medium to give chlorite ion \(\mathrm{\left(\mathrm{ClO}_{2}^{-}\right)}\)and oxygen gas.
The balanced chemical equation for the reaction is
315070
In the redox reaction,
\[\begin{array}{l}
{\rm{xKMn}}{{\rm{O}}_{\rm{4}}} + {\rm{N}}{{\rm{H}}_3} \to {\rm{yKN}}{{\rm{O}}_{\rm{3}}} + {\rm{Mn}}{{\rm{O}}_{\rm{2}}}\\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, + {\rm{KOH}} + {{\rm{H}}_{\rm{2}}}{\rm{O}}
\end{array}\]
x and y are
315106
For the redox reaction
\(\rm{\mathrm{MnO}_{4}^{-}+\mathrm{C}_{2} \mathrm{O}_{4}^{2-}+\mathrm{H}^{+} \rightarrow \mathrm{Mn}^{2+}+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O}}\)
The correct coefficient of the reactants for the balanced equation are
\(\,\,\,\,{\rm{MnO}}_{\rm{4}}^{\rm{ - }}\quad {{\rm{C}}_{\rm{2}}}{\rm{O}}_{\rm{4}}^{{\rm{2 - }}}\quad {{\rm{H}}^{\rm{ + }}}\)
315073
\(\begin{array}{l}
{\rm{F}}{{\rm{e}}^{2 + }} \to {\rm{F}}{{\rm{e}}^{3 + }} + {{\rm{e}}^ - }\\
{\rm{MnO}}_4^ - + {\rm{5}}{{\rm{e}}^ - } \to {\rm{M}}{{\rm{n}}^{2 + }}
\end{array}\)
The ratio of stoichiometric coefficient of \({\rm{F}}{{\rm{e}}^{2 + }}\) and \({\rm{MnO}}_4^ - \)
315076
Dichlorine heptaoxide \(\mathrm{\left(\mathrm{Cl}_{2} \mathrm{O}_{7}\right)}\) in gaseous state combines with an aqueous solution of hydrogen peroxide in acidic medium to give chlorite ion \(\mathrm{\left(\mathrm{ClO}_{2}^{-}\right)}\)and oxygen gas.
The balanced chemical equation for the reaction is
315070
In the redox reaction,
\[\begin{array}{l}
{\rm{xKMn}}{{\rm{O}}_{\rm{4}}} + {\rm{N}}{{\rm{H}}_3} \to {\rm{yKN}}{{\rm{O}}_{\rm{3}}} + {\rm{Mn}}{{\rm{O}}_{\rm{2}}}\\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, + {\rm{KOH}} + {{\rm{H}}_{\rm{2}}}{\rm{O}}
\end{array}\]
x and y are