315010
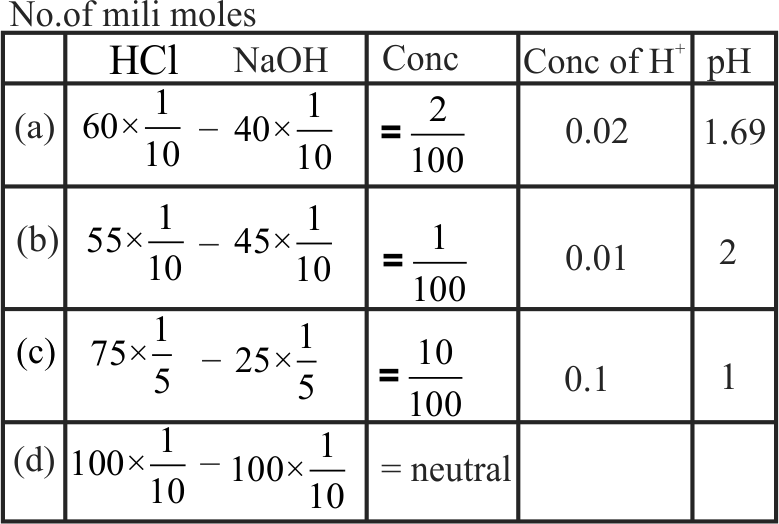
Following solutions were prepared by mixing different volumes of \(\mathrm{NaOH}\) and \(\mathrm{HCl}\) of different concentrations :
(A) \({\text{60 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{HCl + 40 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{NaOH}}\)
(B) \({\text{55 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{HCl + 45 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{NaOH}}\)
(C) \({\text{75 mL}}\frac{{\text{M}}}{{\text{5}}}{\text{HCl + 25 mL}}\frac{{\text{M}}}{{\text{5}}}{\text{NaOH}}\)
(D) \({\text{100 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{HCl + 100 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{NaOH}}\)
\(\mathrm{pH}\) of which one of them will be equal to 1?
315010
Following solutions were prepared by mixing different volumes of \(\mathrm{NaOH}\) and \(\mathrm{HCl}\) of different concentrations :
(A) \({\text{60 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{HCl + 40 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{NaOH}}\)
(B) \({\text{55 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{HCl + 45 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{NaOH}}\)
(C) \({\text{75 mL}}\frac{{\text{M}}}{{\text{5}}}{\text{HCl + 25 mL}}\frac{{\text{M}}}{{\text{5}}}{\text{NaOH}}\)
(D) \({\text{100 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{HCl + 100 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{NaOH}}\)
\(\mathrm{pH}\) of which one of them will be equal to 1?
315010
Following solutions were prepared by mixing different volumes of \(\mathrm{NaOH}\) and \(\mathrm{HCl}\) of different concentrations :
(A) \({\text{60 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{HCl + 40 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{NaOH}}\)
(B) \({\text{55 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{HCl + 45 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{NaOH}}\)
(C) \({\text{75 mL}}\frac{{\text{M}}}{{\text{5}}}{\text{HCl + 25 mL}}\frac{{\text{M}}}{{\text{5}}}{\text{NaOH}}\)
(D) \({\text{100 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{HCl + 100 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{NaOH}}\)
\(\mathrm{pH}\) of which one of them will be equal to 1?
315010
Following solutions were prepared by mixing different volumes of \(\mathrm{NaOH}\) and \(\mathrm{HCl}\) of different concentrations :
(A) \({\text{60 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{HCl + 40 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{NaOH}}\)
(B) \({\text{55 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{HCl + 45 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{NaOH}}\)
(C) \({\text{75 mL}}\frac{{\text{M}}}{{\text{5}}}{\text{HCl + 25 mL}}\frac{{\text{M}}}{{\text{5}}}{\text{NaOH}}\)
(D) \({\text{100 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{HCl + 100 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{NaOH}}\)
\(\mathrm{pH}\) of which one of them will be equal to 1?
315010
Following solutions were prepared by mixing different volumes of \(\mathrm{NaOH}\) and \(\mathrm{HCl}\) of different concentrations :
(A) \({\text{60 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{HCl + 40 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{NaOH}}\)
(B) \({\text{55 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{HCl + 45 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{NaOH}}\)
(C) \({\text{75 mL}}\frac{{\text{M}}}{{\text{5}}}{\text{HCl + 25 mL}}\frac{{\text{M}}}{{\text{5}}}{\text{NaOH}}\)
(D) \({\text{100 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{HCl + 100 mL}}\frac{{\text{M}}}{{{\text{10}}}}{\text{NaOH}}\)
\(\mathrm{pH}\) of which one of them will be equal to 1?