CHXI07:EQUILIBRIUM
314888
Match Column I with Column II and choose the correct combination from the options given
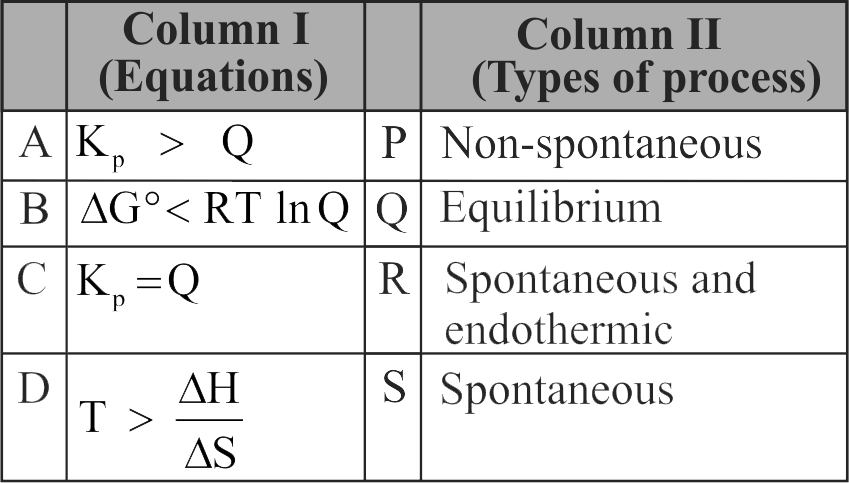
1 \({\text{A}} - {\text{P}},{\mkern 1mu} {\mkern 1mu} {\text{B}} - {\text{Q}},{\mkern 1mu} {\mkern 1mu} {\text{C}} - {\text{R}},{\mkern 1mu} {\mkern 1mu} {\text{D}} - {\text{S}}\)
2 \({\text{A}} - {\text{R}},{\mkern 1mu} {\mkern 1mu} {\text{B}} - {\text{S}},{\mkern 1mu} {\mkern 1mu} {\text{C}} - {\text{Q}},{\mkern 1mu} {\mkern 1mu} {\text{D}} - {\text{P}}\)
3 \({\text{A}} - {\text{S}},{\mkern 1mu} {\mkern 1mu} {\text{B}} - {\text{P}},{\mkern 1mu} {\mkern 1mu} {\text{C}} - {\text{Q}},{\mkern 1mu} {\mkern 1mu} {\text{D}} - {\text{R}}\)
4 \({\text{A}} - {\text{Q}},{\mkern 1mu} {\mkern 1mu} {\text{B}} - {\text{P}},{\mkern 1mu} {\mkern 1mu} {\text{C}} - {\text{S}},{\mkern 1mu} {\mkern 1mu} {\text{D}} - {\text{R}}\)
Explanation:
(1) When \({{\text{K}}_{\text{p}}} > {\text{Q}},\) the reaction goes in forward direction, i.e., the reaction is spontaneous.
(2)\(\Delta {\text{G}} = \Delta {\text{G}}^\circ + {\text{RT}}\,\,{\text{ln}}Q,\Delta {\text{G}}^\circ < {\text{RT}}\,\,{\text{ln}}\,{\text{Q}}\), thus, \(\Delta {\text{G}} = + {\text{ve}}\) and hence, the reaction is non-spontaneous.
(3) At equilibrium, \({{\text{K}}_{\text{p}}} = {\text{Q}}\)
(4) \({\text{T}} > \frac{{\Delta {\text{H}}}}{{\Delta {\text{S}}}}\) or \({\text{T}}\Delta {\text{S}} > \Delta {\text{H}}\)
This condition is true for spontaneous, endothermic reactions \(({\text{as}}\,\,\Delta {\text{G}} = \Delta {\text{H}} - {\text{T}}\Delta {\text{S}})\).