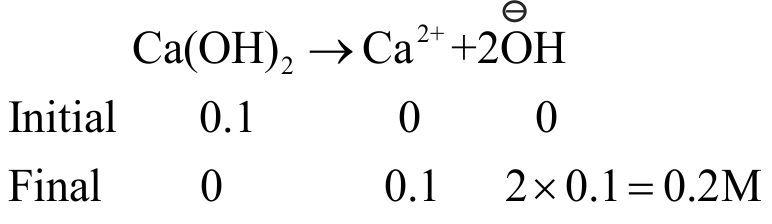314522 pH of an aqueous solution of \({\mathrm{0.6\, \mathrm{M}\, \mathrm{NH}_{3}}}\) and \({\mathrm{0.4 \, \mathrm{M} \, \mathrm{NH}_{4} \mathrm{Cl}}}\) is 9.4 \({\rm{(p}}{{\rm{K}}_{\rm{b}}}{\rm{ = }}\,{\rm{4}}.{\rm{74}})\). The new pH when \({\mathrm{0.1 \mathrm{M} \mathrm{Ca}(\mathrm{OH})_{2}}}\) solution is added to it is
314524
Read the Statement -A and Statement -B carefully to mark the correct options given below:
Statement A :
Mixture of \(\mathrm{CH}_{3} \mathrm{COOH}\) and \(\mathrm{CH}_{3} \mathrm{COONH}_{4}\) is an example of acidic buffer.
Statement B :
Acidic buffer contains equimolar mixture of a weak acid and its salt with strong base.
314522 pH of an aqueous solution of \({\mathrm{0.6\, \mathrm{M}\, \mathrm{NH}_{3}}}\) and \({\mathrm{0.4 \, \mathrm{M} \, \mathrm{NH}_{4} \mathrm{Cl}}}\) is 9.4 \({\rm{(p}}{{\rm{K}}_{\rm{b}}}{\rm{ = }}\,{\rm{4}}.{\rm{74}})\). The new pH when \({\mathrm{0.1 \mathrm{M} \mathrm{Ca}(\mathrm{OH})_{2}}}\) solution is added to it is
314524
Read the Statement -A and Statement -B carefully to mark the correct options given below:
Statement A :
Mixture of \(\mathrm{CH}_{3} \mathrm{COOH}\) and \(\mathrm{CH}_{3} \mathrm{COONH}_{4}\) is an example of acidic buffer.
Statement B :
Acidic buffer contains equimolar mixture of a weak acid and its salt with strong base.
314522 pH of an aqueous solution of \({\mathrm{0.6\, \mathrm{M}\, \mathrm{NH}_{3}}}\) and \({\mathrm{0.4 \, \mathrm{M} \, \mathrm{NH}_{4} \mathrm{Cl}}}\) is 9.4 \({\rm{(p}}{{\rm{K}}_{\rm{b}}}{\rm{ = }}\,{\rm{4}}.{\rm{74}})\). The new pH when \({\mathrm{0.1 \mathrm{M} \mathrm{Ca}(\mathrm{OH})_{2}}}\) solution is added to it is
314524
Read the Statement -A and Statement -B carefully to mark the correct options given below:
Statement A :
Mixture of \(\mathrm{CH}_{3} \mathrm{COOH}\) and \(\mathrm{CH}_{3} \mathrm{COONH}_{4}\) is an example of acidic buffer.
Statement B :
Acidic buffer contains equimolar mixture of a weak acid and its salt with strong base.
314522 pH of an aqueous solution of \({\mathrm{0.6\, \mathrm{M}\, \mathrm{NH}_{3}}}\) and \({\mathrm{0.4 \, \mathrm{M} \, \mathrm{NH}_{4} \mathrm{Cl}}}\) is 9.4 \({\rm{(p}}{{\rm{K}}_{\rm{b}}}{\rm{ = }}\,{\rm{4}}.{\rm{74}})\). The new pH when \({\mathrm{0.1 \mathrm{M} \mathrm{Ca}(\mathrm{OH})_{2}}}\) solution is added to it is
314524
Read the Statement -A and Statement -B carefully to mark the correct options given below:
Statement A :
Mixture of \(\mathrm{CH}_{3} \mathrm{COOH}\) and \(\mathrm{CH}_{3} \mathrm{COONH}_{4}\) is an example of acidic buffer.
Statement B :
Acidic buffer contains equimolar mixture of a weak acid and its salt with strong base.
314522 pH of an aqueous solution of \({\mathrm{0.6\, \mathrm{M}\, \mathrm{NH}_{3}}}\) and \({\mathrm{0.4 \, \mathrm{M} \, \mathrm{NH}_{4} \mathrm{Cl}}}\) is 9.4 \({\rm{(p}}{{\rm{K}}_{\rm{b}}}{\rm{ = }}\,{\rm{4}}.{\rm{74}})\). The new pH when \({\mathrm{0.1 \mathrm{M} \mathrm{Ca}(\mathrm{OH})_{2}}}\) solution is added to it is
314524
Read the Statement -A and Statement -B carefully to mark the correct options given below:
Statement A :
Mixture of \(\mathrm{CH}_{3} \mathrm{COOH}\) and \(\mathrm{CH}_{3} \mathrm{COONH}_{4}\) is an example of acidic buffer.
Statement B :
Acidic buffer contains equimolar mixture of a weak acid and its salt with strong base.