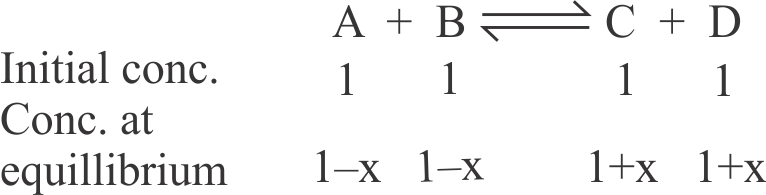314449 In the equilibrium \(2 \mathrm{NH}_{3} \rightleftharpoons \mathrm{N}_{2}+3 \mathrm{H}_{2}, 6\) moles of \(\mathrm{NH}_{3}\) is taken in \(10 \mathrm{~L}\) flask. If concentration of \(\mathrm{N}_{2}\) at equilibrium is x, then concentration of \(\mathrm{NH}_{3}\) at equilibrium is
314450
In a 2 litre flask, the reaction takes place as:
\({\text{COC}}{{\text{l}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{CO(g) + C}}{{\text{l}}_{\text{2}}}{\text{(g)}}\)
The equilibrium conc. of \(\mathrm{COCl}_{2}\) was found to be 0.4. If excess of \(\mathrm{COCl}_{2}\) is added to the system, the equilibrium re-establish and \({\kern 1pt} \left[ {{\text{COC}}{{\text{l}}_{\text{2}}}} \right]\) becomes 1.6. What is the equilibrium conc. of \([\mathrm{CO}]\) ?
314451 The equilibrium constant at 298 K for a reaction \({\mathrm{\mathrm{A}_{(\mathrm{g})}+\mathrm{B}_{(\mathrm{g})} \rightleftharpoons \mathrm{C}_{(\mathrm{g})}+\mathrm{D}_{(\mathrm{g})}}}\) is 100 . If the initial concentration of all the four species were 1 M each, then equilibrium concentration of \({\mathrm{D}}\) (in \({\mathrm{\mathrm{mol} \mathrm{L}^{-1}}}\) ) will be ____ .
314452 \(\mathrm{K}_{\mathrm{c}}\) for \(\mathrm{PCl}_{5}(\mathrm{~g}) \rightleftharpoons \mathrm{PCl}_{3}(\mathrm{~g})+\mathrm{Cl}_{2}(\mathrm{~g})\) is 0.04 at \(250^{\circ} \mathrm{C}\). How many moles of \(\mathrm{PCl}_{5}\) must be added to 3L flask to obtain a \(\mathrm{Cl}_{2}\) concentration of 0.15 M .
314467
\(\mathrm{CuSO}_{4} \cdot 5 \mathrm{H}_{2} \mathrm{O}(\mathrm{s}) \rightleftharpoons\)\(\mathrm{CuSO}_{4} \cdot 3 \mathrm{H}_{2} \mathrm{O}(\mathrm{s})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{g})\)
\(\mathrm{K}_{\mathrm{p}}=1.086 \times 10^{-4} \mathrm{~atm}^{2}\) at \(25^{\circ} \mathrm{C}\). The efflorescent nature of \(\mathrm{CuSO}_{4} \cdot 5 \mathrm{H}_{2} \mathrm{O}\) can be noticed when the vapour pressure of \(\mathrm{H}_{2} \mathrm{O}\) in atmosphere is
314449 In the equilibrium \(2 \mathrm{NH}_{3} \rightleftharpoons \mathrm{N}_{2}+3 \mathrm{H}_{2}, 6\) moles of \(\mathrm{NH}_{3}\) is taken in \(10 \mathrm{~L}\) flask. If concentration of \(\mathrm{N}_{2}\) at equilibrium is x, then concentration of \(\mathrm{NH}_{3}\) at equilibrium is
314450
In a 2 litre flask, the reaction takes place as:
\({\text{COC}}{{\text{l}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{CO(g) + C}}{{\text{l}}_{\text{2}}}{\text{(g)}}\)
The equilibrium conc. of \(\mathrm{COCl}_{2}\) was found to be 0.4. If excess of \(\mathrm{COCl}_{2}\) is added to the system, the equilibrium re-establish and \({\kern 1pt} \left[ {{\text{COC}}{{\text{l}}_{\text{2}}}} \right]\) becomes 1.6. What is the equilibrium conc. of \([\mathrm{CO}]\) ?
314451 The equilibrium constant at 298 K for a reaction \({\mathrm{\mathrm{A}_{(\mathrm{g})}+\mathrm{B}_{(\mathrm{g})} \rightleftharpoons \mathrm{C}_{(\mathrm{g})}+\mathrm{D}_{(\mathrm{g})}}}\) is 100 . If the initial concentration of all the four species were 1 M each, then equilibrium concentration of \({\mathrm{D}}\) (in \({\mathrm{\mathrm{mol} \mathrm{L}^{-1}}}\) ) will be ____ .
314452 \(\mathrm{K}_{\mathrm{c}}\) for \(\mathrm{PCl}_{5}(\mathrm{~g}) \rightleftharpoons \mathrm{PCl}_{3}(\mathrm{~g})+\mathrm{Cl}_{2}(\mathrm{~g})\) is 0.04 at \(250^{\circ} \mathrm{C}\). How many moles of \(\mathrm{PCl}_{5}\) must be added to 3L flask to obtain a \(\mathrm{Cl}_{2}\) concentration of 0.15 M .
314467
\(\mathrm{CuSO}_{4} \cdot 5 \mathrm{H}_{2} \mathrm{O}(\mathrm{s}) \rightleftharpoons\)\(\mathrm{CuSO}_{4} \cdot 3 \mathrm{H}_{2} \mathrm{O}(\mathrm{s})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{g})\)
\(\mathrm{K}_{\mathrm{p}}=1.086 \times 10^{-4} \mathrm{~atm}^{2}\) at \(25^{\circ} \mathrm{C}\). The efflorescent nature of \(\mathrm{CuSO}_{4} \cdot 5 \mathrm{H}_{2} \mathrm{O}\) can be noticed when the vapour pressure of \(\mathrm{H}_{2} \mathrm{O}\) in atmosphere is
314449 In the equilibrium \(2 \mathrm{NH}_{3} \rightleftharpoons \mathrm{N}_{2}+3 \mathrm{H}_{2}, 6\) moles of \(\mathrm{NH}_{3}\) is taken in \(10 \mathrm{~L}\) flask. If concentration of \(\mathrm{N}_{2}\) at equilibrium is x, then concentration of \(\mathrm{NH}_{3}\) at equilibrium is
314450
In a 2 litre flask, the reaction takes place as:
\({\text{COC}}{{\text{l}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{CO(g) + C}}{{\text{l}}_{\text{2}}}{\text{(g)}}\)
The equilibrium conc. of \(\mathrm{COCl}_{2}\) was found to be 0.4. If excess of \(\mathrm{COCl}_{2}\) is added to the system, the equilibrium re-establish and \({\kern 1pt} \left[ {{\text{COC}}{{\text{l}}_{\text{2}}}} \right]\) becomes 1.6. What is the equilibrium conc. of \([\mathrm{CO}]\) ?
314451 The equilibrium constant at 298 K for a reaction \({\mathrm{\mathrm{A}_{(\mathrm{g})}+\mathrm{B}_{(\mathrm{g})} \rightleftharpoons \mathrm{C}_{(\mathrm{g})}+\mathrm{D}_{(\mathrm{g})}}}\) is 100 . If the initial concentration of all the four species were 1 M each, then equilibrium concentration of \({\mathrm{D}}\) (in \({\mathrm{\mathrm{mol} \mathrm{L}^{-1}}}\) ) will be ____ .
314452 \(\mathrm{K}_{\mathrm{c}}\) for \(\mathrm{PCl}_{5}(\mathrm{~g}) \rightleftharpoons \mathrm{PCl}_{3}(\mathrm{~g})+\mathrm{Cl}_{2}(\mathrm{~g})\) is 0.04 at \(250^{\circ} \mathrm{C}\). How many moles of \(\mathrm{PCl}_{5}\) must be added to 3L flask to obtain a \(\mathrm{Cl}_{2}\) concentration of 0.15 M .
314467
\(\mathrm{CuSO}_{4} \cdot 5 \mathrm{H}_{2} \mathrm{O}(\mathrm{s}) \rightleftharpoons\)\(\mathrm{CuSO}_{4} \cdot 3 \mathrm{H}_{2} \mathrm{O}(\mathrm{s})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{g})\)
\(\mathrm{K}_{\mathrm{p}}=1.086 \times 10^{-4} \mathrm{~atm}^{2}\) at \(25^{\circ} \mathrm{C}\). The efflorescent nature of \(\mathrm{CuSO}_{4} \cdot 5 \mathrm{H}_{2} \mathrm{O}\) can be noticed when the vapour pressure of \(\mathrm{H}_{2} \mathrm{O}\) in atmosphere is
314449 In the equilibrium \(2 \mathrm{NH}_{3} \rightleftharpoons \mathrm{N}_{2}+3 \mathrm{H}_{2}, 6\) moles of \(\mathrm{NH}_{3}\) is taken in \(10 \mathrm{~L}\) flask. If concentration of \(\mathrm{N}_{2}\) at equilibrium is x, then concentration of \(\mathrm{NH}_{3}\) at equilibrium is
314450
In a 2 litre flask, the reaction takes place as:
\({\text{COC}}{{\text{l}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{CO(g) + C}}{{\text{l}}_{\text{2}}}{\text{(g)}}\)
The equilibrium conc. of \(\mathrm{COCl}_{2}\) was found to be 0.4. If excess of \(\mathrm{COCl}_{2}\) is added to the system, the equilibrium re-establish and \({\kern 1pt} \left[ {{\text{COC}}{{\text{l}}_{\text{2}}}} \right]\) becomes 1.6. What is the equilibrium conc. of \([\mathrm{CO}]\) ?
314451 The equilibrium constant at 298 K for a reaction \({\mathrm{\mathrm{A}_{(\mathrm{g})}+\mathrm{B}_{(\mathrm{g})} \rightleftharpoons \mathrm{C}_{(\mathrm{g})}+\mathrm{D}_{(\mathrm{g})}}}\) is 100 . If the initial concentration of all the four species were 1 M each, then equilibrium concentration of \({\mathrm{D}}\) (in \({\mathrm{\mathrm{mol} \mathrm{L}^{-1}}}\) ) will be ____ .
314452 \(\mathrm{K}_{\mathrm{c}}\) for \(\mathrm{PCl}_{5}(\mathrm{~g}) \rightleftharpoons \mathrm{PCl}_{3}(\mathrm{~g})+\mathrm{Cl}_{2}(\mathrm{~g})\) is 0.04 at \(250^{\circ} \mathrm{C}\). How many moles of \(\mathrm{PCl}_{5}\) must be added to 3L flask to obtain a \(\mathrm{Cl}_{2}\) concentration of 0.15 M .
314467
\(\mathrm{CuSO}_{4} \cdot 5 \mathrm{H}_{2} \mathrm{O}(\mathrm{s}) \rightleftharpoons\)\(\mathrm{CuSO}_{4} \cdot 3 \mathrm{H}_{2} \mathrm{O}(\mathrm{s})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{g})\)
\(\mathrm{K}_{\mathrm{p}}=1.086 \times 10^{-4} \mathrm{~atm}^{2}\) at \(25^{\circ} \mathrm{C}\). The efflorescent nature of \(\mathrm{CuSO}_{4} \cdot 5 \mathrm{H}_{2} \mathrm{O}\) can be noticed when the vapour pressure of \(\mathrm{H}_{2} \mathrm{O}\) in atmosphere is
314449 In the equilibrium \(2 \mathrm{NH}_{3} \rightleftharpoons \mathrm{N}_{2}+3 \mathrm{H}_{2}, 6\) moles of \(\mathrm{NH}_{3}\) is taken in \(10 \mathrm{~L}\) flask. If concentration of \(\mathrm{N}_{2}\) at equilibrium is x, then concentration of \(\mathrm{NH}_{3}\) at equilibrium is
314450
In a 2 litre flask, the reaction takes place as:
\({\text{COC}}{{\text{l}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{CO(g) + C}}{{\text{l}}_{\text{2}}}{\text{(g)}}\)
The equilibrium conc. of \(\mathrm{COCl}_{2}\) was found to be 0.4. If excess of \(\mathrm{COCl}_{2}\) is added to the system, the equilibrium re-establish and \({\kern 1pt} \left[ {{\text{COC}}{{\text{l}}_{\text{2}}}} \right]\) becomes 1.6. What is the equilibrium conc. of \([\mathrm{CO}]\) ?
314451 The equilibrium constant at 298 K for a reaction \({\mathrm{\mathrm{A}_{(\mathrm{g})}+\mathrm{B}_{(\mathrm{g})} \rightleftharpoons \mathrm{C}_{(\mathrm{g})}+\mathrm{D}_{(\mathrm{g})}}}\) is 100 . If the initial concentration of all the four species were 1 M each, then equilibrium concentration of \({\mathrm{D}}\) (in \({\mathrm{\mathrm{mol} \mathrm{L}^{-1}}}\) ) will be ____ .
314452 \(\mathrm{K}_{\mathrm{c}}\) for \(\mathrm{PCl}_{5}(\mathrm{~g}) \rightleftharpoons \mathrm{PCl}_{3}(\mathrm{~g})+\mathrm{Cl}_{2}(\mathrm{~g})\) is 0.04 at \(250^{\circ} \mathrm{C}\). How many moles of \(\mathrm{PCl}_{5}\) must be added to 3L flask to obtain a \(\mathrm{Cl}_{2}\) concentration of 0.15 M .
314467
\(\mathrm{CuSO}_{4} \cdot 5 \mathrm{H}_{2} \mathrm{O}(\mathrm{s}) \rightleftharpoons\)\(\mathrm{CuSO}_{4} \cdot 3 \mathrm{H}_{2} \mathrm{O}(\mathrm{s})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{g})\)
\(\mathrm{K}_{\mathrm{p}}=1.086 \times 10^{-4} \mathrm{~atm}^{2}\) at \(25^{\circ} \mathrm{C}\). The efflorescent nature of \(\mathrm{CuSO}_{4} \cdot 5 \mathrm{H}_{2} \mathrm{O}\) can be noticed when the vapour pressure of \(\mathrm{H}_{2} \mathrm{O}\) in atmosphere is