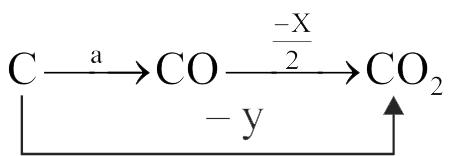369458
Given
(A) \(2{\text{C}}{{\text{O}}_{({\text{g}})}} + {{\text{O}}_{2(\;{\text{g}})}} \to \) \(2{\text{C}}{{\text{O}}_{2(\;{\text{g}})}}\Delta {\text{H}}{^\circ _1} = - {\text{x}}\,\,{\text{kJ}}\,\,{\text{mo}}{{\text{l}}^{ - 1}}\)
(B) \({{\text{C}}_{{\text{(graphitc) }}}} + {{\text{O}}_{2(\;{\text{g}})}} \to \) \({\text{C}}{{\text{O}}_{2(\;{\text{g}})}}\Delta {\text{H}}{^\circ _2} = - {\text{y}}\,\,{\text{kJ}}\,\,{\text{mo}}{{\text{l}}^{ - 1}}\)
The \(\Delta {\text{H}}^\circ \) for the reaction
\({{\text{C}}_{{\text{(graphite) }}}} + \frac{1}{2}{{\text{O}}_{2(\;{\text{g}})}} \to {\text{C}}{{\text{O}}_{({\text{g}})}}\) is
369458
Given
(A) \(2{\text{C}}{{\text{O}}_{({\text{g}})}} + {{\text{O}}_{2(\;{\text{g}})}} \to \) \(2{\text{C}}{{\text{O}}_{2(\;{\text{g}})}}\Delta {\text{H}}{^\circ _1} = - {\text{x}}\,\,{\text{kJ}}\,\,{\text{mo}}{{\text{l}}^{ - 1}}\)
(B) \({{\text{C}}_{{\text{(graphitc) }}}} + {{\text{O}}_{2(\;{\text{g}})}} \to \) \({\text{C}}{{\text{O}}_{2(\;{\text{g}})}}\Delta {\text{H}}{^\circ _2} = - {\text{y}}\,\,{\text{kJ}}\,\,{\text{mo}}{{\text{l}}^{ - 1}}\)
The \(\Delta {\text{H}}^\circ \) for the reaction
\({{\text{C}}_{{\text{(graphite) }}}} + \frac{1}{2}{{\text{O}}_{2(\;{\text{g}})}} \to {\text{C}}{{\text{O}}_{({\text{g}})}}\) is
369458
Given
(A) \(2{\text{C}}{{\text{O}}_{({\text{g}})}} + {{\text{O}}_{2(\;{\text{g}})}} \to \) \(2{\text{C}}{{\text{O}}_{2(\;{\text{g}})}}\Delta {\text{H}}{^\circ _1} = - {\text{x}}\,\,{\text{kJ}}\,\,{\text{mo}}{{\text{l}}^{ - 1}}\)
(B) \({{\text{C}}_{{\text{(graphitc) }}}} + {{\text{O}}_{2(\;{\text{g}})}} \to \) \({\text{C}}{{\text{O}}_{2(\;{\text{g}})}}\Delta {\text{H}}{^\circ _2} = - {\text{y}}\,\,{\text{kJ}}\,\,{\text{mo}}{{\text{l}}^{ - 1}}\)
The \(\Delta {\text{H}}^\circ \) for the reaction
\({{\text{C}}_{{\text{(graphite) }}}} + \frac{1}{2}{{\text{O}}_{2(\;{\text{g}})}} \to {\text{C}}{{\text{O}}_{({\text{g}})}}\) is
369458
Given
(A) \(2{\text{C}}{{\text{O}}_{({\text{g}})}} + {{\text{O}}_{2(\;{\text{g}})}} \to \) \(2{\text{C}}{{\text{O}}_{2(\;{\text{g}})}}\Delta {\text{H}}{^\circ _1} = - {\text{x}}\,\,{\text{kJ}}\,\,{\text{mo}}{{\text{l}}^{ - 1}}\)
(B) \({{\text{C}}_{{\text{(graphitc) }}}} + {{\text{O}}_{2(\;{\text{g}})}} \to \) \({\text{C}}{{\text{O}}_{2(\;{\text{g}})}}\Delta {\text{H}}{^\circ _2} = - {\text{y}}\,\,{\text{kJ}}\,\,{\text{mo}}{{\text{l}}^{ - 1}}\)
The \(\Delta {\text{H}}^\circ \) for the reaction
\({{\text{C}}_{{\text{(graphite) }}}} + \frac{1}{2}{{\text{O}}_{2(\;{\text{g}})}} \to {\text{C}}{{\text{O}}_{({\text{g}})}}\) is
369458
Given
(A) \(2{\text{C}}{{\text{O}}_{({\text{g}})}} + {{\text{O}}_{2(\;{\text{g}})}} \to \) \(2{\text{C}}{{\text{O}}_{2(\;{\text{g}})}}\Delta {\text{H}}{^\circ _1} = - {\text{x}}\,\,{\text{kJ}}\,\,{\text{mo}}{{\text{l}}^{ - 1}}\)
(B) \({{\text{C}}_{{\text{(graphitc) }}}} + {{\text{O}}_{2(\;{\text{g}})}} \to \) \({\text{C}}{{\text{O}}_{2(\;{\text{g}})}}\Delta {\text{H}}{^\circ _2} = - {\text{y}}\,\,{\text{kJ}}\,\,{\text{mo}}{{\text{l}}^{ - 1}}\)
The \(\Delta {\text{H}}^\circ \) for the reaction
\({{\text{C}}_{{\text{(graphite) }}}} + \frac{1}{2}{{\text{O}}_{2(\;{\text{g}})}} \to {\text{C}}{{\text{O}}_{({\text{g}})}}\) is