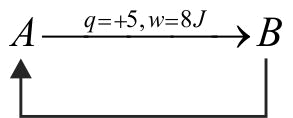369388 A gas undergoes change from state A to state B. In this process, the heat absorbed and work done by the gas is \(\mathrm{5 \mathrm{~J}}\) and \(\mathrm{8 \mathrm{~J}}\), respectively. Now gas is brought back to A by another process during which \(\mathrm{3 \mathrm{~J}}\) of heat is evolved. In this reverse process of \(\mathrm{\mathrm{B}}\) to \(\mathrm{\mathrm{A}}\).
369388 A gas undergoes change from state A to state B. In this process, the heat absorbed and work done by the gas is \(\mathrm{5 \mathrm{~J}}\) and \(\mathrm{8 \mathrm{~J}}\), respectively. Now gas is brought back to A by another process during which \(\mathrm{3 \mathrm{~J}}\) of heat is evolved. In this reverse process of \(\mathrm{\mathrm{B}}\) to \(\mathrm{\mathrm{A}}\).
369388 A gas undergoes change from state A to state B. In this process, the heat absorbed and work done by the gas is \(\mathrm{5 \mathrm{~J}}\) and \(\mathrm{8 \mathrm{~J}}\), respectively. Now gas is brought back to A by another process during which \(\mathrm{3 \mathrm{~J}}\) of heat is evolved. In this reverse process of \(\mathrm{\mathrm{B}}\) to \(\mathrm{\mathrm{A}}\).
369388 A gas undergoes change from state A to state B. In this process, the heat absorbed and work done by the gas is \(\mathrm{5 \mathrm{~J}}\) and \(\mathrm{8 \mathrm{~J}}\), respectively. Now gas is brought back to A by another process during which \(\mathrm{3 \mathrm{~J}}\) of heat is evolved. In this reverse process of \(\mathrm{\mathrm{B}}\) to \(\mathrm{\mathrm{A}}\).