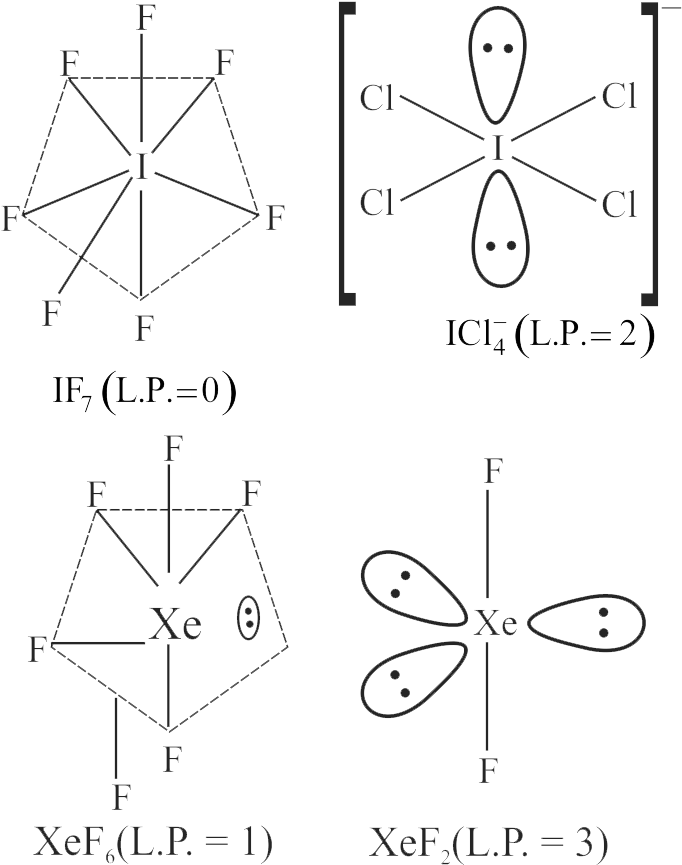313978
Consider the following statements
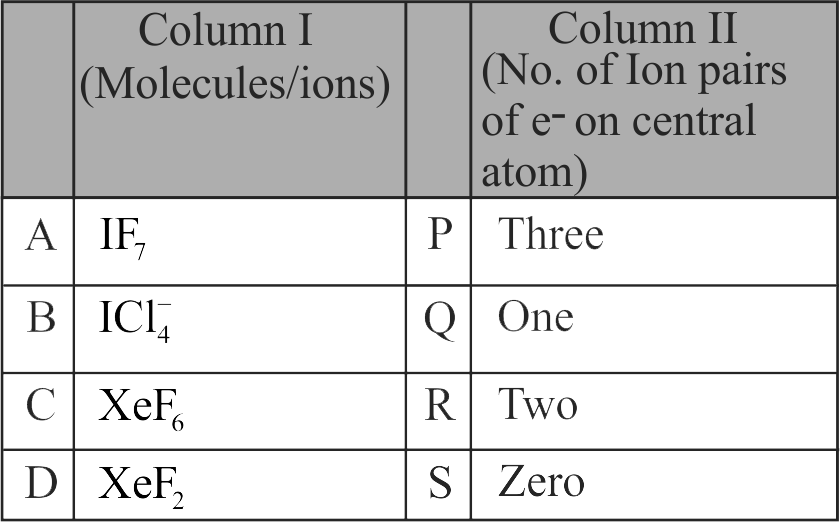
I. \({{\rm{H}}_{\rm{2}}}{\rm{S}}\) molecule has V-shape with two lone pairs of electrons.
II. \({\rm{Xe}}{{\rm{F}}_{\rm{2}}}\) molecule has linear shape with three lone pair of electrons.
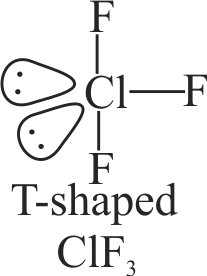
III. \({\rm{Cl}}{{\rm{F}}_{\rm{3}}}\) is T-shaped molecule with two lone pairs of electrons.
Which of the above statements are correct?
Choose the correct option.
313979
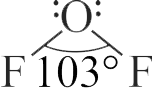
For \(\mathrm{OF}_2\) molecule consider the following:
I. Number of lone pairs on oxygen is 2 .
II. FOF angle is less than \(104.5^{\circ}\)
III. Oxidation state of O is \( - \)2 .
IV. Molecule is bent or ' V ' shaped.
V. Molecular geometry is linear.
Correct statements are
313981
The geometry with respect to the central atom of the following molecules are :
\({\rm{N}}{\left( {{\rm{Si}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{3}}}{\rm{;M}}{{\rm{e}}_{\rm{3}}}{\rm{N;}}{\left( {{\rm{Si}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{3}}}{\rm{P}}\)
313978
Consider the following statements
I. \({{\rm{H}}_{\rm{2}}}{\rm{S}}\) molecule has V-shape with two lone pairs of electrons.
II. \({\rm{Xe}}{{\rm{F}}_{\rm{2}}}\) molecule has linear shape with three lone pair of electrons.
III. \({\rm{Cl}}{{\rm{F}}_{\rm{3}}}\) is T-shaped molecule with two lone pairs of electrons.
Which of the above statements are correct?
Choose the correct option.
313979
For \(\mathrm{OF}_2\) molecule consider the following:
I. Number of lone pairs on oxygen is 2 .
II. FOF angle is less than \(104.5^{\circ}\)
III. Oxidation state of O is \( - \)2 .
IV. Molecule is bent or ' V ' shaped.
V. Molecular geometry is linear.
Correct statements are
313981
The geometry with respect to the central atom of the following molecules are :
\({\rm{N}}{\left( {{\rm{Si}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{3}}}{\rm{;M}}{{\rm{e}}_{\rm{3}}}{\rm{N;}}{\left( {{\rm{Si}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{3}}}{\rm{P}}\)
313978
Consider the following statements
I. \({{\rm{H}}_{\rm{2}}}{\rm{S}}\) molecule has V-shape with two lone pairs of electrons.
II. \({\rm{Xe}}{{\rm{F}}_{\rm{2}}}\) molecule has linear shape with three lone pair of electrons.
III. \({\rm{Cl}}{{\rm{F}}_{\rm{3}}}\) is T-shaped molecule with two lone pairs of electrons.
Which of the above statements are correct?
Choose the correct option.
313979
For \(\mathrm{OF}_2\) molecule consider the following:
I. Number of lone pairs on oxygen is 2 .
II. FOF angle is less than \(104.5^{\circ}\)
III. Oxidation state of O is \( - \)2 .
IV. Molecule is bent or ' V ' shaped.
V. Molecular geometry is linear.
Correct statements are
313981
The geometry with respect to the central atom of the following molecules are :
\({\rm{N}}{\left( {{\rm{Si}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{3}}}{\rm{;M}}{{\rm{e}}_{\rm{3}}}{\rm{N;}}{\left( {{\rm{Si}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{3}}}{\rm{P}}\)
313978
Consider the following statements
I. \({{\rm{H}}_{\rm{2}}}{\rm{S}}\) molecule has V-shape with two lone pairs of electrons.
II. \({\rm{Xe}}{{\rm{F}}_{\rm{2}}}\) molecule has linear shape with three lone pair of electrons.
III. \({\rm{Cl}}{{\rm{F}}_{\rm{3}}}\) is T-shaped molecule with two lone pairs of electrons.
Which of the above statements are correct?
Choose the correct option.
313979
For \(\mathrm{OF}_2\) molecule consider the following:
I. Number of lone pairs on oxygen is 2 .
II. FOF angle is less than \(104.5^{\circ}\)
III. Oxidation state of O is \( - \)2 .
IV. Molecule is bent or ' V ' shaped.
V. Molecular geometry is linear.
Correct statements are
313981
The geometry with respect to the central atom of the following molecules are :
\({\rm{N}}{\left( {{\rm{Si}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{3}}}{\rm{;M}}{{\rm{e}}_{\rm{3}}}{\rm{N;}}{\left( {{\rm{Si}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{3}}}{\rm{P}}\)
313978
Consider the following statements
I. \({{\rm{H}}_{\rm{2}}}{\rm{S}}\) molecule has V-shape with two lone pairs of electrons.
II. \({\rm{Xe}}{{\rm{F}}_{\rm{2}}}\) molecule has linear shape with three lone pair of electrons.
III. \({\rm{Cl}}{{\rm{F}}_{\rm{3}}}\) is T-shaped molecule with two lone pairs of electrons.
Which of the above statements are correct?
Choose the correct option.
313979
For \(\mathrm{OF}_2\) molecule consider the following:
I. Number of lone pairs on oxygen is 2 .
II. FOF angle is less than \(104.5^{\circ}\)
III. Oxidation state of O is \( - \)2 .
IV. Molecule is bent or ' V ' shaped.
V. Molecular geometry is linear.
Correct statements are
313981
The geometry with respect to the central atom of the following molecules are :
\({\rm{N}}{\left( {{\rm{Si}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{3}}}{\rm{;M}}{{\rm{e}}_{\rm{3}}}{\rm{N;}}{\left( {{\rm{Si}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{3}}}{\rm{P}}\)